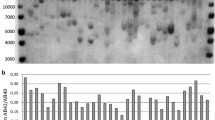
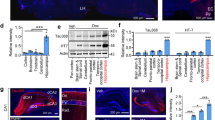
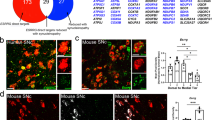
Abstract
Nonregulatable promoters have been mainly used to produce transgenic mice that express the human genes for Alzheimer's disease (AD). The aim of this study was to produce doubly transgenic mice expressing the regulatable tet promoter-controlled transactivator (tTA) and human mutant presenilin 2 (N141I, hPS2m) genes in order to examine the AD-related phenotypes at the basal and inducible levels. To achieve this, the first lineage of the transgenic line, expressing Tet/tTA and the second lineage of transgenic mice, expressing Tet/hPS2m, were created, and the doubly transgenic mice were produced by crossing the Tet/tTA-transgenic mice with the Tet/hPS2m-transgenic mice. The doubly transgenic mice and nontransgenic littermates were then treated with or without doxycycline. The results showed that removing doxycycline from the transgenic mice resulted in the induction of the transgene, a Wnt signaling defect, behavioral impairment, elevated amyloid-β-42 and γ-secretase activity compared with in the group given doxycyline. Moreover, the expression levels of the hPS2m transgene decreased gradually in the transgenic males, with clear changes becoming apparent between 2 and 4 wk of age. Castrating these males resulted in an increased expression level of the hPS2m gene. This was restored to the normal levels by treatment with testosterone. Therefore, tetregulated transgenic mice can be used to examine the effect of the basal or inducible expression levels of hPS2m on the pathology of AD at the “on/off” states at any stage of development.
Similar content being viewed by others
References
Borchelt D. R., Thinakaran G., Eckman C. B., et al. (1996) Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ-40 ratio in vitro and in vivo. Neuron 17, 1005–1013.
Citron M., Westaway D., Xia W., et al. (1997) Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β protein in both transfected cell and transgenic mice. Nat. Med. 3, 67–72.
Corder E. H., Saunders A. M., Strittmatter W. J., et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 828–829.
Dale T. C. (1998) Signal transduction by Wnt family of ligands. Biochem. J. 15, 209–223.
De Ferrari G. V., Chacon M. A., Barria M. I., et al. (2003) Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta amyloid fibrils. Mol. Psychiatry 8, 195–208.
De Strooper B., Annaert W., Cupers P., et al. (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch interacellular domain. Nature 398, 518–522.
Doble B. W. and Woodgett J. R. (2003) GSK3: tricks of the trade for a multitasking kinase. J. Cell Sci. 116, 1175–1186.
Duff K., Eckman C., Zehr C., et al. (1996) Increased amyloid β-42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713.
Forss S., Danielson P. E., Catsicas S., et al. (1990) Transgenic mice expressing β-galactosidase in mature neuron under neuron-specific enolase promoter control. Cell 5, 187–197.
Hwang D. Y., Cho J. S., Chae K. R., et al. (2003) Differential expression of the tetracycline-controlled transactivator-driven human CYP1B1 gene in double-transgenic mice is due to androgens: application for detecting androgens and antiandrogens. Arch. Biochem. Biophys. 415, 137–145.
Hwang D. Y., Chae K. R., Kang T. S., et al. (2002) Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 16, 805–813.
Hwang D. Y., Cho J. S., Lee S. H., et al. (2004) Aberrant expressions of pathogenic phenotype in Alzheimer's diseased transgenic mice carrying NSE-controlled APPsw. Exp. Neurol. 186, 20–32.
Janicki S. and Monteiro M. J. (1997) Increased apoptosis arising from increased expression of the Alzheimer's disease-associated presenilin-2 mutation (N141I). J. Cell Biol. 139, 485–495.
Kim Y. K. and Ogita Z. (1981) Sexual dimorphism of SDS-peptide patterns from submaxillary gland of mice. J. Exp. Zool. 218, 447–453.
Kim T. W., Pettingell W. H., Hallmark O. G., et al. (1997a) Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J. Biol. Chem. 272, 11,006–11,010.
Kim T. W., Pettingell W. H., Jung Y. K., et al. (1997b) Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277, 373–376.
Kuo Y. M., Emmerling M. R., Vigo-Pelfrey C., et al. (1996) Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J. Biol. Chem. 271, 4077–4081.
Levy-Lahad E., Wasco W., Poorkaj P., et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269, 973–977.
Lim H. J., Cho J. S., Oh J. H., et al. (2005) NSE-controlled carboxyl-terminus of APP gene overex pressing in transgenic mice induces altered expression in behavior, Aβ-42, and GSK3β binding proteins. Cell Mol. Neurobiol. 25, 833–850.
Loetscher H., Deuschle U., Brockhaus M., et al. (1997) Presenilins are processed by caspase-type proteases. J. Biol. Chem. 272, 20,655–20,659.
Lue L. F., Kuo Y. M., Roher A. E., et al. (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am. J. Pathol. 155, 853–862.
Martin D. I. and Whitelaw E. (1996) The vagaries of variegating transgenes. Bioassay 18, 919–923.
McLean C. A., Cherny R. A., Fraser F. W., et al. (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 46, 860–866.
Morris R. G. M., Garrud P., Rawlins J. N. P., et al. (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683.
Murayama M., Tanake S., Palacino J., et al. (1998) Direct association of presenilin-1 with beta-catenin. FEBS Lett. 433, 73–77.
Naslund J., Haroutunian V., Moshs R., et al. (2000) Correlation between elevated levels of amyloid betapeptide in the brain and cognitive decline. J. Am. Med. Assoc. 283, 1571–1577.
Nusse R. (1999) WNT targets. Repression and activation. Trends Genet. 15, 1–3.
Oyama F., Sawamura N., Kobayashi K., et al. (1998) Mutant presenilin 2 transgenic mouse: effect on an age-dependent increase of amyloid beta-protein 42 in the brain. J. Neurochem. 71, 313–322.
Rogaev E. I., Sherrington R., Rogaeva E. A., et al. (1995) Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 376, 775–778.
Sawamura N., Morishima-Kawashima M., Waki H., et al. (2000) Mutant presenilin 2 transgenic mice. A large increase in the levels of Abeta 42 is presumably associated with the low density membrane domain that contains decreased levels of glycerophospholipids and sphingomyelin. J. Biol. Chem. 275, 27,901–27,908.
Scheuner D., Eckman C., Jensen M., et al. (1996) Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870.
Sherrington R., Rogaev E., Liang Y., et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760.
Selkoe D. J. and Wolfe M. S. (2000) In search of gammasecretase: presenilin at the cutting edge. Proc. Natl. Acad. Sci. USA 97, 5690–5692.
Sisodia S. S., Annaert W., Kim S. H., et al. (2001) Gamma-secretase: never more enigmatic. Trends Neurosci. 24, 2–6.
Takashima A., Honda T., Yasutake K., et al. (1998a) Activation of tau protein kinase I/glycogen synthasekinase-3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neurosci. Res. 31, 317–323.
Takashima A., Murayama M., Murayama O., et al. (1998b) Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc. Natl. Acad. Sci. USA 95, 9637–9641.
Tsien J. Z., Huerta P. T., and Tonegawa S. (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338.
Walker E. S., Martinez M., Brunkan A. L., et al. (2005) Presenilin 2 familial Alzheimer's disease mutation in partial loss of function and dramatic changes in Abeta 42/40 ratios. J. Neurochem. 92, 294–301.
Xia W., Ostaszewski B. L., Kimberly W. T., et al. (2000) FAD mutations in presenilin-1 or amyloid precursor protein decrease the efficacy of a gammasecretase inhibitor: evidence for direct involvement of PS1 in the gamma-secretase cleavage complex. Neurobiol. Dis. 7, 673–681.
Yu G., Chen F., Levesque G., et al. (1998) The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta catenin. J. Biol. Chem. 273, 16,470–16,475.
Zhang Z., Hartmann H., Do V. M., et al. (1998) Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395, 698–702.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, D.Y., Cho, J.S., Kim, C.K. et al. Changes in presenilin 2-binding Wnt proteins, behavior, amyloid-β 42, γ-secretase activity, and testosterone sensitivity in transgenic mice coexpressing tetracycline-controlled transactivator and human mutant presenilin 2. Neuromol Med 8, 415–431 (2006). https://doi.org/10.1385/NMM:8:3:415
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:8:3:415