Abstract
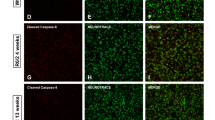
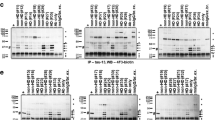
There is increasing evidence implicating apoptosis-mediated cell death in the pathogenesis of neurodegenerative diseases. One important event in the apoptotic cascade is the release of cytochrome c by mitochondria into the cytoplasm, activating caspase-9, leading to the subsequent activation of downstream executioner caspases. In the present study, we examined the distribution of cytochrome c and caspase-9 in Huntington’s disease (HD) patients and in a transgenic model of HD (R6/2 line). Neuronal cytochrome c immunoreactivity increased with neuropathological severity in HD patients. Concomitant with this finding, Western-blot analysis showed a shift in the distribution of cytochrome c from the mitochondrial to the cytosolic fraction with incremental cytosolic expression associated with greater striatal degeneration. Active caspase-9 immunoreactivity was present in both HD striatal neurons and in Western blots of severe-grade specimens. Similar findings were observed in the R6/2 mice. There was a temporal increase in expression and shift of cytochrome c from the mitochondrial to the cytosolic fraction from 4–13 wk of age. Activated caspase-9 and caspase 3 activities were present only at endstage disease. Although the present results provide evidence that key components of the intrinsic mitochondrial apoptotic pathway are activated in both HD patients and a transgene murine model of HD, these phenomena are prominent in only severe neuropathological grades in HD patients and HD mice, suggesting that apoptosis may play a greater role in neuronal death at endstage disease.
Similar content being viewed by others
References
Beal M. F. (2000) Energetics in the pathogenesis of neurodegenerative diseases. TINS 23, 298–304.
Buki A., Okonkwo D. O., Wang K. K. W., and Povlishock J. T. (2000) Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci. 20, 2825–2834.
Cha J. H. (2000) Transcriptional dysregulation in Huntington’s disease. TINS 23, 387–392.
Chen Q., Gong B., and Almasan A. (2000) Distinct stages of cytochrome c release from mitochondria: Evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7, 227–233.
Chen M., Ona V. O., Li M., Ferrante R. J., Fink K. B., Zhu S., et al. (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 6, 797–801.
Chu Z. L., Pio F., Xie Z., Welsh K., Krajewska M., Krajewski S., Godzik A., and Reed J. C. (2001) A novel enhancer of the Apaf1 Apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J. Biol. Chem. 276, 9239–9245.
Cohen G. M. (1997) Caspases: The executioners of apoptosis. J. Biochem. 326, 1–16.
Desagher S. and Martinou J. C. (1998) Mitochondria as the central point of apoptosis. Trends Cell Biol. 10, 369–377.
Dragunow M., Faull R. L., Lawlor P., Beilharz E. J., Singleton K., Walker E. B., and Mee E. (1995) In situ evidence for DNA fragmentation in Huntington’s disease striatum and Alzheimer’s disease temporal lobes. Neuroreport 6, 1053–1057.
Du C., Fang M., Li Y., Li L., and Wang X. (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42.
Ferrante R. J., Andreassen O. A., Jenkins B. G., Dedeoglu A., Kuemmerle S., Kubilus J. K., et al. (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 20, 4389–4397.
Fujimura M., Morita-Fujimura Y., Muratami K., Kawase M., and Chan P. H. (1998) Cytosolic redistribution of cytochrome c after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 18, 1239–1247.
Goldberg Y. P., Nicholson D. W., Rasper D. M., Kalchman M. A., Koide H. B., Graham R. K., et al. (1996) Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet 13, 442–449.
Green D. R. and Reed J. C. (1998) Mitochondria and apoptosis. Science 281, 1309–1312.
Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72, 971–983.
Kowall N. W. and Ferrante R. J. (1998) Huntington’s disease, in Neuropathology of Dementing Disorders (Markesbery M., ed.), Edward Arnold, London, UK, pp. 219–256.
Krajewska M., Wang H. G., Krajewski S., Zapata J. M., Shabaik A., Gascoyne R., and Reed J. C. (1997) Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 57, 1605–1613.
Kuemmerle S., Gutekunst C. A., Klein A. M., Li X. J., Li S. H., Beal M. F., et al. (1999) Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann. Neurol. 46, 842–849.
La Spada A. R., Paulson H. L., and Fischbeck K. H. (1994) Trinucleotide repeat expansion in neurological disease Ann. Neurol. 36, 814–822.
Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., and Wang X.. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489.
Li S. H., Lam S., Cheng A. L., and Li X. J. (2000) Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum. Mol. Genet. 9, 2859–2867.
Liu X., Kim C. N., Yang J., Jemmerson R., and Wang X. (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157.
Mattson M. P. and Duan W. (1999) “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res. 58, 152–166.
Miyashita T., Matsui J., Ohtsuka Y., Mami U., Fujishima S., Okamura-Oho Y., et al. (1999) Expression of extended polyglutamine sequentially activates initiator and effector caspases. Biochem. Biophys. Res. Commun. 257, 724–730.
Offen D., Elkon H., and Melamed E. (2000) Apoptosis as a general cell death pathway in neurodegenerative diseases. J. Neural Transm. Suppl. 58, 153–166.
Ona V. O., Li M., Vonsattel J. P., Andrews L. J., Khan S. Q., Chung W. M., et al. (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399, 263–267.
Pasinelli P., Houseweart M. K., Brown R. H., and Cleveland D. W. (2000) Caspase-1 and -3 are sequentially activated in motor neuron death in CuZn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 97, 13901–13906.
Plassart E. and Fontaine B. (1994) Genes with triplet repeats: a new class of mutations causing neurological diseases. Biomed. Pharmacother. 48, 191–197.
Petersen A., Mani K., and Brundin P. (1999) Recent advances on the pathogenesis of Huntington’s disease. Exp. Neurol. 157, 1–18.
Portera-Cailliau C., Hedreen J. C., Price D. L., and Koliatsos V. E. (1995) Evidence for apoptotic cell death in Huntington’s disease and excitotoxic animal models. J. Neurosci. 15, 3775–3787.
Qin Z. H., Wang Y., Kikly K. K., Sapp E., Kegel K. B., Aronin N., and DiFiglia M. (2001) Pro-caspase-8 is predominately localized in mitochondria and released into cytoplasm upon apoptotic stimulation. J. Biol. Chem. 276, 8079–8086.
Rey L, and Maier R.J. (1997) Cytochrome c terminal oxidase pathways of Azotobacter vinelandii: analysis of cytochrome c4 and c5 mutants and up-regulation of cytochrome c-dependent pathways with N2 fixation. J. Bacteriol. 179, 7191–7196.
Rigamonti D., Bauer J. H., De-Fraja C., Conti L., Sipione S., Sciorati C., et al. (2000) Wild-type Huntingtin protects from apoptosis upstream of caspase-3. J. Neurosci. 20, 3705–3713.
Ross C. A. (1997) Intranuclear neuronal inclusions: a common pathogenic mechanism for glutamine-repeat neurodegenerative diseases? Neuron 19, 1147–1150.
Roy S. and Nicholson D. W. (2000) Programmed cell-death regulation: basic mechanism and therapeutic opportunities. Mol. Med. Today 6, 264–266.
Sanchez I., Xu C. J., Juo P., Kazizaka A., Blenis J., and Yuan J. (1999) Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22, 623–633.
Sawa A., Wiegand G. W., Cooper J., Margolis R. L., Sharp A. H., Lawler J. F. Jr, et al. (1999) Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nature Med. 5, 1194–1198.
Shimohama S. (2000) Apoptosis in Alzheimer’s disease: an update. Apoptosis 5, 9–16.
Thornberry N. and Lazebnik Y. (1998) Caspases: enemies within. Science 281, 1312–1316.
Turmaine M., Raza A., Mahal A., Mangiarini L., Bates G. P., and Davies S. W. (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 97, 8093–8097.
Vonsattel J. P., Myers R. H., Stevens T. J., Ferrante R. J., Bird E. D., and Richardson E. P. Jr. (1985) Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 44, 559–577.
Vukosavic S., Stefanis L., Jackson-Lewis V., Guegan C., Romero N., Chen C., et al. (2000) Delaying caspase activation by Bcl-2: A clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 20, 9119–9125.
Wellington C. L., Singaraja R., Ellerby L., Savill J., Roy S., Leavitt B., et al. (2000) Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J. Biol. Chem. 275, 19831–19838.
Wyllie A. H., Kerr J. F., and Currie A. R. (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol. 68, 251–306.
Zou H., Li Y., Liu X., and Wang X. (1999) An APAF-1/cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274, 11549–11556.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiechle, T., Dedeoglu, A., Kubilus, J. et al. Cytochrome C and caspase-9 expression in Huntington’s disease. Neuromol Med 1, 183–195 (2002). https://doi.org/10.1385/NMM:1:3:183
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:1:3:183