Abstract
Introduction
Cerebral vasculopathy may play an important role in the development of delayed cerebral ischemia following subarachnoid hemorrhage (SAH). Platelet-derived growth factor AB (PDGF-AB) and vascular endothelial growth factor (VEGF) released from blood clot may trigger vasculopathy in cerebral arteries. We compared arteriographic and histological response to injection of blood with PDGF-AB and VEGF in basilar artery over 3 days.
Methods
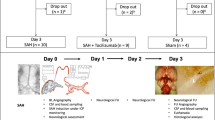
A total of 55 male New Zealand white rabbits were used in this study. SAH was simulated by single injection of 1 ml of autologous blood, while 50 μg PDGF-AB, 100 μg VEGF, and 50 μg PDGF-AB/100 μg VEGF combined were given in autologous cerebrospinal fluid (CSF) into the cisterna magna. Eight rabbits served as controls, receiving arteriograms but no cisternal injections, and 13 rabbits received reinjections of CSF. Basilar artery diameter was measured arteriographically at baseline and 3 days after injection. Immunohistochemistry was performed to assess change in the basilar artery.
Results
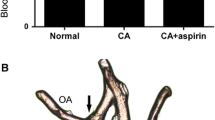
Control groups showed significantly less (p<0.0002) basilar artery narrowing than all treatment groups. Proliferating cell nuclear antigen (PCNA) was not significant for treatment groups compared to CSF reinject control. PCNA was significantly different for the no puncture group compared to the CSF reinject control. Von Willebrand Factor staining may indicate endothelial proliferation in the adventitia of SAH, VEGF, and PDGF-AB/VEGF combined groups. PDGF labeling was abundant for SAH, PDGF-AB, and PGDF-AB/VEGF combined.
Conclusions
PDGF-AB/VEGF and VEGF cause narrowing of the basilar artery similar to cisternal blood clot at 3 days, and thus blood clot was not required to cause arteriographic changes consistent with cerebral vasculopathy.
Similar content being viewed by others
References
Macdonald R. Pathophysiology and molecular genetics of vasospasm. Acta Neurochir Suppl 2001;77:7–11.
Rabinstein A, Pichelmann M, Friedman J, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg 2003;98:319–325.
Mayberg MR. Cerebral vasospasm. Neurosurg Clin N Am 1998;9:615–627.
Dorsch N. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care 2002;8:128–133.
Mayberg M, Okada T, Bark D. Morphologic changes in cerebral arteries after subarachnoid hemorrhage. Neurosurg Clin N Am 1990;1:417–429.
Graves E. Detailed diagnoses and procedures. National Hospital Discharge Survey, 1990, 1992; Vital Health Statistics 13:113–225.
Raines EW, Ross R. Platelet-derived growth factor in vivo. In: Westermark B, Sorg C, ed. Biology of platelet-derived growth factor. Basel: Karger, 1993.
Plate KH, Warnke PC. Vascular endothelial growth factor. J Neurooncol 1997;35:365–372.
Borel C, McKee A, Parra A, et al. A possible role for vascular cell proliferation in cerebral vasospasm following subarachnoid hemorrhage. Stroke 2003;34:427–433.
Pollay M, Davson H. The passage of certain substances out of the cerebrosphinal fluid. Brain 1963;86:137–150.
Zhang A. Broad-spectrum and selective serine protease inhibitors prevent expression of platelet-derived growth factor-bb and cerebral vasospasm after subarachnoid hemorrhage. Stroke 2001;32:1665–1672.
Masson PJ. J Technol Methods 1929;12:75.
Lindman HR, ed. Analysis of variance in experimental design. New York: Springer-Verlag, 1992.
Montgomery DC, ed. Design and analysis of experiments. New York: John Wiley & Sons Inc, 1997.
Vorkapic P, Bevan R, Bevan J. Longitudinal time course of reversible and irreversible components of chronic cerebrovasospasm of the rabbit basilar artery. J Neurosurg 1991;74:951–955.
Salgado R, Benoy I, Bogers J, et al. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis 2001;4:37–43.
Schwartz SM, deBlois D, O'Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res 1995;77:445–465.
Takenaka K, Goto Y, Kassell N, Lee K. Modification of vascular smooth muscle proteins in rabbit basilar artery after subarachnoid hemorrhage. In: Findlay J, ed. Cerebral vasospasm. Amsterdam: Elsevier, 1993, pp. 93–96.
Vorkapic P, Bevan R, Bevan J. Pharmacologic irreversible narrowing in chronic cerebrovasospasm in rabbits is associated with functional damage. Stroke 1990;21:1478–1484.
Megyesi J, Vollrath B, Cook D, Findlay J. In vivo animal models of cerebral vasospasm: a review. Neurosurg 2000;46:448–461.
Lin CL, Jeng AY, Howng SL, Kwan AL. Endothelin and subarachnoid hemorrhage-induced cerebral vasospasm: pathogenesis and treatment. Curr Med Chem 2004;11:1779–1791.
Faraci F, Heistad D. Regulation of cerebral circulation: role of endothelium and potassium channels. Physiol Rev 1998;78:53–97.
Takenaka K, Kishino J, Yamada H, et al. DNA synthesis and intracellular calcium elevation in porcine cerebral arterial smooth muscle cells by cerebrospinal fluid from patients with subarachnoid haemorrhage. Neurol 1992;14:330–334.
Smith R, Clower B, Grotendorst G, Yabuno N, Cruse J. Arterial wall changes in early human vasospasm. Neurosurg 1985;16:171–176.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, C.A., Lombard, F.W., Wu, CT. et al. Role of vascular mitogens in subarachnoid hemorrhage-associated cerebral vasculopathy. Neurocrit Care 5, 215–221 (2006). https://doi.org/10.1385/NCC:5:3:215
Issue Date:
DOI: https://doi.org/10.1385/NCC:5:3:215