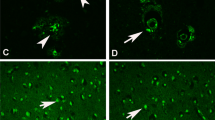
Abstract
Zinc is one of the most abundant transition metals in the brain. A substantial fraction (10–15%) of brain zinc is located inside presynaptic vesicles of certain glutamatergic terminals in a free or loosely bound state. This vesicle zinc is released with neuronal activity or depolarization, probably serving physiologic functions. However, with excess release, as may occur in a variety of pathologic conditions, zinc may translocate to and accumulate in postsynaptic neurons, events which may contribute to selective neuronal cell death. Intracellular mechanisms of zinc neurotoxicity may include disturbances in energy metabolism, increases in oxidative stress, and activation of apoptosis cascades. Zinc inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and depletes nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP). On the other hand, zinc activates protein kinase C (PKC) and extracellular signal-regulated kinase (Erk-1/2), and induces NADPH oxidase; these events results in oxidative neuronal injury. Zinc can also trigger caspase activation and apoptosis via the p75NTR pathway. Interestingly, the converse—depletion of intracellular zinc—also induces neuronal death, but in this case, exclusively via classical apoptosis. In addition to the neurotoxic effect, zinc may contribute to the pathogenesis of chronic neurodegenerative disease. For example, in Alzheimer’s disease (AD), mature amyloid plaques, but not preamyloid deposits, are found to contain high levels of zinc, suggesting the role of zinc in the process of plaque maturation. Further insights into roles of zinc in brain diseases may help set a new direction toward the development of effective treatments.
Similar content being viewed by others
References
Keilin D. and Mann T. (1939) Carbonic anhydrase. Nature 144, 442.
Coleman J. E. (1992) Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 61, 897–946.
Vallee B. L. and Falchuck K. H. (1993) The biochemical basis of zinc physiology. Physiol. Rev. 73, 79–118.
Vallee B. L. and Auld D. S. (1995) Metallochemistry in biochemistry. EXS 73, 259–277.
O’Halloran T. V. (1993) Transition metals in control of gene expression. Science 261, 715–725.
Vallee B. L., Coleman J. E., and Auld D. S. (1991) Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. USA 88, 999–1003.
Szallasi Z., Bogi K., Gohari S., Biro T., Acs P., and Blumberg P. M. (1996) Non-equivalent role for the first and second zinc fingers of protein kinase Cdelta. Effect of their mutation of phorbol ester-induced translocation in NIH 3T3 cells. J. Biol. Chem. 271, 18,229–18,301.
Sakane F., Yamada K., Kanoh H., Yokoyama C., and Tanabe T. (1990) Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 344, 345–348.
Mills J. S. and Johnson J. D. (1985) Metal ions as allosteric regulators of calmodulin. J. Biol. Chem. 260, 15,100–15,105.
Baudier J., Haglid K., Haiech J., and Gerard D. (1983) Zinc ion binding to human brain calcium binding proteins, calmodulin and S 100b protein. Biochem. Biophys. Res. Commun. 114, 1138–1146.
Ebadi M., Elsayed M. A., and Aly M. H. (1994) The importance of zinc and metallothionein in the brain. Biol. Signals. 3, 123–126.
Aschner M., Cherian M. G., Klassen C. D. Palmiter R. D., Erickson J. C., and Bush A. I. (1997) Metallothioneins in the brain: the role in physiology and pathology. Toxicol. Appl. Pharmacol. 142, 229–242.
Wallwork J. C. (1987) Zinc and the central nervous system. Prog. Food Nutr. Sci. 11, 203–247.
Frederickson C. J. (1989) Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 31, 145–238.
Frederickson C. J., Klitenick M. A., Manton W. I., and Kirkpatrick J. B. (1983) Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 273, 335–339.
Danscher G. (1981) Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry 71, 1–16.
Frederickson C. J., Kasarskis E. J., Ringo D., Frederickson R. E. (1987) A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods 20, 91–103.
Wenzel H. J., Cole T. B., Born D. E., Schwartzkroin P. A., and Palmiter R. D. (1997) Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 94, 12,676–12,681.
Perez-Clausell J. (1996) Distribution of terminal fields stained for zinc in the neocortex of the rat. J. Chem. Neuroanat. 11, 99–111.
Sensi S. L., Canzoniero L. M. T., Yu S. P., Ying H., Koh J. Y., Kerchner G. A., and Choi D. W. (1997) Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 17, 9554–9564.
Howell G. A., Welch M. G., and Frederickson C. J. (1984) Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 308, 736–738.
Assaf S. Y. and Chung S. H. (1984) Release of endogenous Zn2+ from brain tissue during activity. Nature 308, 734–736.
Choi D. W. and Koh J. H. (1998) Zinc and brain injury. Annu. Rev. Neurosci. 21, 347–375.
Tonder N, Johansen F. F., Frederickson C. J., Zimmer J., and Diemer N. H. (1990) Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 109, 247–252.
Koh J. Y., Suh S. W., Gwag B. J., He Y. Y., Hsu C. Y., and Choi D. W. (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272, 1013–1016.
Lee J. M., Zipfel G. J., and Choi D. W. (1999) The changing landscape of ischaemic brain injury mechanisms. Nature 399, A7–14.
Frederickson C. J., Hernandez M. D., and McGinty J. F. (1989) Translocation of zinc may contribute to seizure-induced death of neurons. Brain Res. 480, 317–321.
Lee J. Y., Cole T. B., Palmiter R. D., and Koh J. Y. (2000) Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J. Neurosci. 20, RC79.
Cole T. B., Wenzel H. J., Kafer K. E., Schwartzkroin P. A., and Palmiter R. D. (1999) Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 96, 1716–1721.
Yeiser E. C., Lerant A. A., Casto R. M., and Levenson C. W. (1999) Free zinc increases at the site of injury after cortical stab wounds in mature but not immature rat brain. Neurosci. Lett. 277, 75–78.
Aizenman E., Stout A. K., Hartnett K. A., Dineley K. E., McLaughlin B., and Reynolds I. J. (2000) Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release: J. Neurochem. 75, 1878–1888.
Suh S. W., Koh J. Y., and Choi D. W. (1996) Extracellular zinc mediates selective neuronal death in hippocampus and amygdala following kainate-induced seizures. Soc. Neurosci. Abstr. 22, 2101.
Lee J. Y., Park J., Kim Y. H., Kim D. H., Kim C. G., and Koh J. Y. (2000) Induction by synaptic zinc of heat shock protein-70 in hippocampus after kainate seizures. Exp. Neurol. 161, 433–441.
Suh S. W., Chen J. W., Motamedi M., Bell B., Listiak K., Pons N. F., et al. (2000) Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 852, 268–273.
Sheline C. T., Behrens M. M., and Choi D. W. (2000) Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD+ and inhibition of glycolysis. J. Neurosci. 20, 3139–3146.
Noh K. M., Kim Y. H., and Koh J. Y. (1999) Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J. Neurochem. 72, 1609–1616.
Park J. A. and Koh J. Y. (1999) Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. J. Neurochem. 73, 450–456.
Kim Y. H., Kim E. Y., Gwag B. J., Sohn S., and Koh J. Y. (1999) Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free-radicals. Neuroscience 89, 175–182.
Noh K. M. and Koh J. Y. (2000) Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci. 20, RC111.
Szabo C. and Dawson V. L. (1998) Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci. 19, 287–298.
Park J. A., Lee, J. Y., Sato T. A., and Koh J. Y. (2000) Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J. Neurosci. 20, 9096–9103.
Mukai J., Hachiya T., Shoji-Hoshino S., Kimura M., Nadano D., Suvanto P., et al. (2000) NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J. Biol. Chem. 275, 17,566–17,570.
Ahn Y. H., Kim Y. H., Hong S. H., and Koh J. Y. (1998) Depletion of intracellular zinc induces protein synthesis-dependent neuronal apoptosis in mouse cortical culture. Exp. Neurol. 154, 47–56.
Weiss J. H., Sensi S. L., and Koh J. Y. (2000) Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 21, 395–401.
Kim A. H., Sheline C. T., Tian M., Higashi T., McMahon R. J., Cousins R. J., and Choi D. W. (2000) L-type Ca2+ channel-mediated Zn2+ toxicity and modulation by ZnT-1 in PC12 cells. Brain Res. 886, 99–107.
Kim Y. H., Park J. H., Hong S. H., and Koh J. Y. (1999) Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science 284, 647–650.
Kim Y. H. and Koh J. Y. (2000) EGF receptor-dependent cytoprotection by tPA and HGF against zinc toxicity in cortical culture. Soc. Neurosci. (Abstr.) 26, 775.
Hyun H. J., Sohn J. H., Ahn Y. H., Shin H. C., Koh J. Y., and Yoon Y. H. (2000) Depletion of intracellular zinc induces macromolecule synthesis- and caspase- dependent apoptosis of cultured retinal cells. Brain Res. 869, 39–48
Hyun H. J., Sohn J. H., HA D. W., Koh J. Y., and Yoon Y. H. (2000) Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest. Opthalmol. Vis. Sci. 42, 460–465.
Constantinidis J. (1991) The hypothesis of zinc deficiency in the pathogenesis of neurofibrillary tangles. Med. Hypotheses 35, 319–323.
Perry D. K., Smyth M. J., Sennicke H. R., Salvesen G. S., Duriez P., Poirier G. G., and Human Y. A. (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 272, 18,530–18,533.
Newsome D. A., Swartz M., Leone N. C., Elston R. C., and Miller E. (1998) Oral zinc in macular degeneration. Arch. Ophthalmol. 106, 192–198.
Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., and Markesbery W. R. (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 158, 47–52.
Suh S. W., Jensen K. B., Jensen M. S., Silva D. S., Kesslak P. J., Danscher G., and Frederickson C. J. (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 852, 274–278.
Lee J. Y., Mook-Jung I. H., and Koh J. Y. (1999) Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J. Neurosci. 19, RC10, 1–5.
Bush A. I., Pettingell W. H., Multhaup G., d Paradis M., Vonsattel J. P., Gusella J. F., et al. (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265, 1464–1467.
Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., et al. (1996) Correlative memory dificits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102.
Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., and Cotman C. W. (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 13, 16676–1687.
Cuajungco M. P., Goldstein L. E., Nunomura A., Smith M. A., Lim J. T., Atwood C. S., et al. (2000) Evidence that the beta-amyloid plaques of Alzheimer’s disease represent the redox-silencing and entombment of abeta by zinc. J. Biol. Chem. 275, 19,439–19,442.
Cherny R. A., Barnham K. J., Lynch T., Volitakis I., Li Q. X., McLean C. A., et al. (2000) Chelation and intercalation: complementary properties in a compound for the treatment of Alzheimer’s disease. J. Struct. Biol. 130, 209–216.
Ha H. C. and Snyder S. H. (2000) Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol. Dis. 7, 225–239.