Abstract
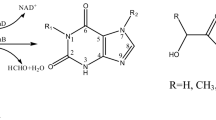
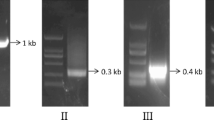
The complementary deoxyribonucleic acid (cDNA) coding spinach glycolate oxidase (GO) was amplified by reverse transcription polymerase chain reaction (RT-PCR), using the total ribonucleic acid (RNA) of spinach leaves as the template, and was cloned into cloning vector pMD18-T. After the DNA sequence was determined, the go gene was subcloned into Escherichia coli expression vectors. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that recombinant GO was expressed in E. coli BL21 (DE3)(pTIG-Trx-GO) and E. coli BL21 (DE3)(pET-22b(+)-GO). The result of the enzyme activity assay and the oxidation of glycolate to glyoxylate catalyzed by the whole cells of E. coli BL21 (DE3)(pET-22b(+)-GO) proved the feasibility of using E. coli cells to produce glyoxylic acid.
Similar content being viewed by others
References
Anton, D. and DiCosimo, R. (1994) Production of glyoxylic acid by oxidizing glycolic acid in the presence of immobilized glycolate oxidase and catalase. Patent WO 94/06925, 31 March, 3–4.
Clagett, C. O., Tolbert, N. E., and Burris, R. H. (1949) Oxidation of α-hydroxy acids by enzymes from plants. J. Biol. Chem. 181, 905–914.
Macheroux, P., Mulrooney, S. B., Williams, C. H., Jr., and Massey, V. (1992) Direct expression of active spinach glycolate oxidase in Escherichia coli. Biochim. Biophys. Acta 1132, 11–16.
Macheroux, P., Massey, V., and Thiele, D. J. (1991) Expression of spinach glycolate oxidase in Saccharomyces cerevisiae: purification and characterization. Biochemistry 30, 4612–4619.
Anton, D. and DiCosimo, R. (1995) Glycolate oxidase production. Patent WO 95/01444, 12 Jan, 5–8
Gellissen, G., Piontek, M., and Dahlems, U. (1996) Recombinant Hansenula polymorpha as a biocatalyst: coexpression of the spinach glycolate oxidase (GO) and the S. cerevisiae catalase T (CTT1) gene. Appl. Microbiol. Biotechnol. 46, 46–54.
Payne, M. S., Petrillo, K. L., and Gavagan, J. E. (1997) Engineering Pichia pastoris for biocatalysis: coproduction of two active enzymes. Gene 194, 179–182.
Garagan, J. E., Fager, S. K., and Seip, J. (1995) Glyoxylic acid production using microbial transformant catalysts. J. Org. Chem. 60, 3957–3963.
Wood C, D. M. et al. (1989) Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 16, 3469–3478.
Studier, F. W., Rosenberg, A. H., Dunn, J. J., and Dubendorff, J. W. (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185, 60–89.
Zhang, D. Z., Wu, S. H., Zhang, Z. Q., et al. (1990) High expression of tumor necrosis factor alpha cDNA in E. coli using site-specific deletion mutagenesis. Chin. J. Biotechnol. 6, 157–164.
Zhao, Z. H., Zhang, Y. S., Liu, C. X., and Ma, Q. J. (2000) A new strategy for soluble expression of foreign proteins in E. coli Biotechnol. Lett. 11, 164.
Stenberg, K. and Lindqvist, Y. (1996) High-level expression, purification, and crystallization of recombinant spinach glycolate oxidase in Escherichia coli. Protein Expr. Purif. 8, 295–298.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Ausubel, F. M. (1995) Current Protocols in Molecular Biology, 3rd ed., Publishing Associates and Wiley Interscience, New York.
Lord, M. J. and Merrett, M. J. (1968) Glycolate oxidase in Chlorella pyrenoidosa. Biochim. Biophys. Acta 159, 543–544.
Volokita, M. and Somervillet, R. C. (1987) The primary structure of spinach glycolate oxidase from the DNA sequence of a cDNA clone. J. Biol. Chem. 262, 15,825–15,828.
Williams, E., Cregeen, D., and Rumsby, G. (2000) Identification and expression of a cDNA for human glycolate oxidase. Biochim. Biophys. Acta 1493, 246–248.
Gough, S., Deshpande, M., Scher, M., Rosazza, J. N. P. (2001) Permeabilization of Pichia pastoris for glycolate oxidase activity. Biotechnol. Letts. 23, 1535–1537.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, J., Tan, T., Wang, H. et al. The expression of spinach glycolate oxidase (GO) in E. coli and the application of GO in the production of glyoxylic acid. Mol Biotechnol 25, 207–214 (2003). https://doi.org/10.1385/MB:25:3:207
Issue Date:
DOI: https://doi.org/10.1385/MB:25:3:207