Abstract
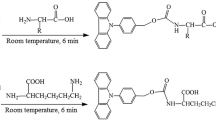
New precolumn derivatizing reagents for analysis of amino acids by HPLC—butylisothiocyanate (BITC) and benzylisothiocyanate (BZITC)—reacted quantitatively with 22 standard amino acids and the amino acids in the acid hydrolysate of food and protein standard, bovine serum albumin (BSA), at 40°C for 30 min to yield butylthiocarbamyl (BTC) amino acids and at 50°C for 30 min to yield benzylthiocarbammyl (BZTC) amino acids. BTC and BZTC amino acids were successfully separated in 35 min on the reversed-phase Nova-Pak C18 column (30 cm × 3.9 mm, 4 µm). The optimum wavelengths for determination of BTC and BZTC derivatives were 240 nm and 246 nm, respectively. Analysis of the results obtained with BSA and food samples as BTC and BZTC derivatives showed good agreement with those determined as ion-exchange chromatography and data presented in the literature. The advantage of BITC reagent over the phenylisothiocyanate (PITC) and BZITC was that it had high volatility, so the excess reagent and by-products were easily removed in about 10 min, compared to about 1 h in the PITC and BZITC reagents. In the BTC and BZTC derivatives, cystine and cysteine were determined separately, but in the PTC amino acids derivatized with PITC reagent they were resolved into single peak.
Similar content being viewed by others
References
White, J. A. and Hart, R. T. (1992) Derivatization methods for liquid chromatographic separation of amino acid, in Food Analysis by HPLC (Nollet, L. M. L., ed.), Marcel Dekker, Inc. New York, pp. 53–74.
Bidingmeyer, B. A., Cohex, S. A., and Tarvin, T. L. (1984) Rapid analysis of amino acids using precolumn derivatization. J. Chromatogr. 336, 93–104.
Heinrikson, R. L. and Meredith, S. C. (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography; precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 136, 65–74.
White, J. A., Hart, R. L., and Fry, L. C. (1986) An evaluation of the Waters Pico-tag system for the amino acid analysis of food materials. J. Auto. Chem. 8, 170–177.
Beaver, R. W., Wilson, D. M., Johes, H. M., and Haydon, K. D. (1987) Amino acid analysis in foods and feedstuffs using precolumn phenylisothiocyanate derivatization and liquid chromatography-precolumn study. J. Ass. Off. Anal. Chem. 70, 425–428.
Bidingmeyer, B. A., Cohen, S. A., Tarvin, T. L., and Frost, B. (1987) A new, rapid, high-sensitivity analysis of amino acids in food type samples. J. Ass. Off. Anal. Chem. 70, 241–247.
Cohen, S. A. and Strydom, D. J. (1988) Amino acid analysis utilizing phenylisothiocyanate derivatives. Anal. Biochem. 174, 1–16.
Koop. D. R., Morgan, E. T., Tarr, G. E., and Coon. M. J. (1982) Purification and characterization of a unique isozyme of cytochrome p-450 from live microsomes of ethanol-treated rabbits. J. Biol. Chem. 257, 8472–8480.
Roth, M. (1971) Fluorescence reaction for amino acids. Anal. Chem. 43, 880–882.
Yaegaki, K., Tonzetich, J., and Ng, A. S. K. (1986) Improved high-performance liquid chromatography method for quantitation of proline and hydroxyproline in biological materials. J. Chromatog. 356, 163–170.
Tapuhi, Y., Schmidt, D. E., Lindner, W., and Karger, B. L. (1981) Dansylation of amino acids for high-performance liquid chromatography analysis. Anal. Biochem. 115, 123–129.
DeJong, C., Hughes, G. J., Wieringen, E. V., and Wilson, K. J. (1982) Amino acid analysis by high-performance liquid chromatography. An evolution of usefulness of pre-column Dns derivatization. J. Chromatogr. 241, 345–359.
Lin, J. K. and Chang, J. Y. (1975) Chromophoric labeling of amino acids with 4-dimethylaminoazobenzene-4′-sulfonyl chloride. Anal. Chem. 47, 1634–1638.
Knecht, R. and Chang, J. Y. (1986) Liquid chromatographic determination of amino acids after gas-phase hydrolysis and derivatization with (dimethylamino)-azobenzene-sulfonyl chloride. Anal. Chem. 58, 2375–2378.
Cohen, S. A. (1990) Analysis of amino acids by liquid chromatography after precolumn derivatization with 4-nitrophenylisothiocyanate. J. Chromatogr. 512, 283–290.
Woo, K. L. and Lee, S. H. (1994) Determination of protein amino acids as butylthiocarbamyl derivatives by reversed-phase high-performance liquid chromatography with precolumn derivatization and UV detection. J. Chromatogr. A. 667, 105–111.
Woo, K. L. Hwang, Q. C., and Kim, H. S. (1996) Determination of amino acids in the foods by reversed-phase high-preformance liquid chromatography with a new precolumn derivative, butylthiocarbamyl amino acid, compared to the conventional phenylthiocarbamyl derivatives and ion-exchange chromatography. J. Chromatogr. A. 740, 31–40.
Woo, K. L. and Ahan, Y. K. (1996) Determination of protein amino acid as benzylthiocarbamyl derivatives compared with phenylthiocarbamyl derivatives by reversed-phase high-performance liquid chromatography, ultraviolet detection and precolumn derivatization. J. Chromatogr. A. 740, 41–50.
Woo, K. L. and Lee, D. S. (1995) Capillary gas chromatographic determination of proteins and biological amino acids as N(o)-tert-butyldimethysilyl derivatives. J. Chromatogr. B. 665, 15–25.
Greenstein, J. P. and Winitz, M. (1986) Chemistry of Amino Acid, vol. 3. Robert E. Krieger Publish., Malabar, FL, p. 1882.
Aitken, A., Geisow, M. J., Findlay, J. B. C., Holmes, C., and Yarwoord, A. (1989) Peptide Preparation and Approach. IRC, Oxford, pp. 43–68.
Fürst, P., Pollack, L., Graser, T. A., Gldel, H., and Stehle, J. (1990) Appraisal of precolumn derivatization methods for the high-performance liquid chromatographic determination of free amino acids in bilogical materials. J. Chromatogr. 499, 559–569.
Dayhoff, M. O. (1976) Atlas of Protein Sequence and Structure. vol. 5, Suppl. 2, Natl. Biomed. Res. Found., Washington, DC, pp. 267.
King, T. P. and Spencer, M. (1970) Structural studies and organic ligand-binding properties of bovine plasma albumin. J. Biol. Chem. 245, 6134–6148.
J. R. Brown (1975) Structure of bovine serum albumin. Fed. Proc. 34, 591.
Hirayama, K., Akashi, S., Furuya, M., and Fukuhara, K. I. (1990) Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun. 173, 639–646.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woo, KL. Determination of amino acids in foods by reversed-phase HPLC with new precolumn derivatives, butylthiocarbamyl, and benzylthiocarbamyl derivatives compared to the phenylthiocarbamyl derivative and ion exchange chromatography. Mol Biotechnol 24, 69–88 (2003). https://doi.org/10.1385/MB:24:1:69
Issue Date:
DOI: https://doi.org/10.1385/MB:24:1:69