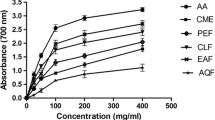
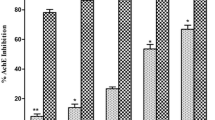
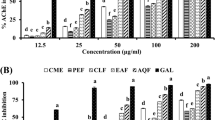
Abstract
Prunus persica L. Batsch water extract (PPE) is a potent acetylcholinesterase (AChE) inhibitor screened for the treatment of Alzheimer's disease. The effects of oral administration of the PPE were examined with comparison of those of selective butyrylcholinesterase inhibitors of 9-amino-1,2,3,4-tetrahydroacridine hydrochloride (tacrine) and tetraidopropylpyrophosphoramide (iso-OMPA) and a selective AChE inhibitor, donepezil, on the cholinesterase activity in the brain and plasma of rats. After the sequential solvent fractionation of the methanol extract of P. persica L. Batsch, the highest inhibitory fraction was that of chloroform (75%). The concentration that was required for 50% enzyme inhibition (IC50 value) was 5.6 μg/mL. for the chloroform fraction. Oral administration of PPE or tacrine caused a dose-dependent inhibition of brain and plasma cholinesterase activities. The ID50 values of these compounds for brain cholinesterase activity were 2.7 g/kg and 8.9 mg/kg, respectively. On the other hand, the ID50 values for plasma cholinesterase activity were 18.6 g/kg and 27.5 mg/kg, respectively. Thus, the ratios of the ID50 (plasma<brain) were 6.0 and 3.1, respectively. These results suggest that orally administered PPE satisfactorily penetrates into the brain and inhibits cholinesterase there and that PPE is a potent inhibitor of brain cholinesterase in comparison with plasma cholinesterase in vivo.
Similar content being viewed by others
References
Akgur S. A., Ozturk P., Sozmen E. Y., Delen Y., Tanyalcin T., and Ege B. (1999) Paraoxonase and acetylcholinesterase activities in humans exposed to organophos-phorous compounds, J. Toxicol. Environ. Health 58, 467–474.
Cohen R. and Barenholz Y. (1984) Characterization of the association of Electrophorus electricus acetyl-cholinesterase with sphingomyelin liposomes. Relevance to collagen-sphingomyelin interactions. Biochim. Biophys. Acta 778, 94–104.
Davis P. and Maloney A. J. (1976) Selective loss of central cholinergic neurons in Alzheimer disease. Lancet 2(8000), 1403.
Drachman D. A. (1977) Memory and cognitive function in man: does the cholinergic system have a specific role. Neurology 27, 783–790.
Edwards J. A. and Brimijoin S. (1982) Divergent regulation of acetylcholinesterase and butyrylcholinesterase in tissues of the rat. J. Neurochem. 38, 1393–1403.
Ellman G. L., Courtney D., Andres V. Jr. and Featherstone R. M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95
Fang X. D. (1986) Active protein constituents from the kernel of Prunus persica. Zhong Yao Tong Bao 11(11), 37–39.
Forette F., Anand R., and Gharabawi G. (1999) A phase II study in patients with Alzheimer's disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine (Exelon). Eur. J. Neurol. 6, 423–429.
Fuhrman B., Partoush A., and Aviram M. (2004) Acetylcholine esterase protects LDL against oxidation. Biochem. Biophys. Res. Commun. 322(3), 974–978
Gilani A. H., Aziz N., Ali S. M., and Saeed M. (2000) Pharmacological basis for the use of peach leaves in constipation. J. Ethnopharmacol. 73, 87–93.
Hass M. A., Nowak D. M., Leonova E., Levin R. M., and Longhurst P. A. (1999) Identification of components of Prunus africana extract that inhibit lipid peroxidation. Phytomedicine 6(5), 379–388.
Heo J., 1991 Dong-eui-bo-gam. Nam-san-dang Publishing Co. Seoul, Korea. p. 712.
Kim H. J., Lee W. H., Yoon C. H., Jeong J. C., Nam K. S., Kim H. M., et al. (2001). Bombycis corpus extract prevents amyloid-beta-induced cytotoxicity and protects superoxide dismutase activity in cultured rat astrocytes. Pharmacol. Res. 43, 11–16.
Kosasa T., Kuriya Y., Matsu K., and Yamanishi Y. (2000) Inhibitory effect of orally administered tacrine, a novel treatment for Alzheimer's disease, on cholinesterase activity in rats. Eur. J. Pharmacol. 389, 173–179.
Menache R., Kenda L., Shaked P., Schwartzman S., and Lewinski U. (1982) The prognostic value of serum acetylcholinesterase in myocardial infarction. Theoretical and clinical considerations. Res. Exp. Med. (Berl.) 181, 181–187.
Nordberg A. and Svensson A. L. (1998) Cholinesterase inhibitors in the treatment of Alzheimer's disease: a comparison of tolerability and pharmacology. Drug Safety 19, 465–480.
Perry E. K., Tomlinson B. E., Blessed G., Bergma K., Gibson P. H., and Perry R. H. (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 2, 1456–1459.
Rogers S. L., Farlow M. R., Doody R. S., Mohs R., and Friedhoff L. T. (1998) A 24-week, double-blind, placebocontrolled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology 50, 136–145.
Shen D., Shen L., and Wang A. L. (1994) Effect of xiaoyu pain on new platelet aggregation defect. Zhongguo Zhong Xi Yi Jie He Za Zhi 14, 589–591.
Sherman K. A. (1991) Pharmacodynamics of oral E2020 and tacrine in humans: novel approaches, in Cholinergic Basis for Alzheimer Therapy, Becker, R., and Giacobini, E., eds. Birkhauser, Boston, MA, pp. 321–328.
Summers W. K., Majovski L. V., Marsh, G. M., Tachiki K., and Kling A. (1986) Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer's type. N. Engl. J. Med. 315, 1241–1245.
Weiner L., Kreimer D., Roth E., and Silman I. (1994) Oxidative stress transforms acetylcholinesterase to a moltenglobule-like state. Biochem. Biophys. Res. Commun. 198, 915–922.
White P., Hiley C. R., Goodhardt M. J., et al. (1977) Neocortical cholinergic neurons in elderly people. Lancet 1(8013) 668–671.
Wilcock G. K., Lilienfeld S., and Gaens E. (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. Br. Med. J. 321, 1445–1449.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suh, SJ., Koo, BS., Jin, UH. et al. Pharmacological characterization of orally active cholinesterase inhibitory activity of Prunus persica L. Batsch in rats. J Mol Neurosci 29, 101–107 (2006). https://doi.org/10.1385/JMN:29:2:101
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:29:2:101