Abstract
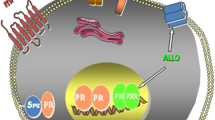
In addition to its traditional role in reproduction, progesterone (PROG) has demonstrated neuroprotective and promyelinating effects in lesions of the peripheral and central nervous systems, including the spinal cord. The latter is a target of PROG, as nuclear receptors, as well as membrane receptors, are expressed by neurons and/or glial cells. When spinal cord injury (SCI) is produced at the thoracic level, several genes become sensitive to PROG in the region caudal to the lesion site. Although the cellular machinery implicated in PROG neuroprotection is only emerging, neurotrophins, their receptors, and signaling cascades might be part of the molecules involved in this process. In rats with SCI, a 3-d course of PROG treatment increased the mRNA of brain-derived neurotrophic factor (BDNF) and BDNF immunoreactivity in perikaryon and processes of motoneurons, whereas chromatolysis was strongly prevented. The increased expression of BDNF correlated with increased immunoreactivity for the BDNF receptor TrkB and for phosphorylated cAMP-responsive element binding in motoneurons. In the same SCI model, PROG restored myelination, according to measurements of myelin basic protein (MBP) and mRNA levels, and further increased the density of NG2 +-positive oligodendrocyte progenitors. These cells might be involved in remyelination of the lesioned spinal cord. Interestingly, similarities in the regulation of molecular parameters and some cellular events attributed to PROG and BDNF (i.e., choline acetyltransferase, Na,K-ATPase, MBP, chromatolysis) suggest that BDNF and PROG might share intracellular pathways. Furthermore, PROG-induced BDNF might regulate, in a paracrine or autocrine fashion, the function of neurons and glial cells and prevent the generation of damage.
Similar content being viewed by others
References
Acheson A. and Linsday R. M. (1996) Non-target-derived roles of the neurotrophins. Phil. Trans. R. Soc. Lond. B Biol. Sci. 351, 417–422.
Afshari F. S., Chu A. K., and Sato-Bigbeee C. (2001) Effect of cyclic AMP on the expression of myelin basic protein species and myelin proteolipid protein in committed oligodeodrocytes: differential involvement of the transcription factor CREB. J. Neurosci. Res. 66, 37–45.
Al-Majed A. A., Brushart T. M., and Gordon T. (2000) Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 12, 4381–4390.
Ankeny D. P., McTigue D. M., Guan Z., Yan Q., Kinstler O., Stokes B. T., and Jakeman L. B. (2001) Pegylafed brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Exp. Neurol. 170, 85–100.
Azcoitia J., Leonelli E., Magnaghi V., Veiga S., García-Segura L. M., and Melcangi R. C. (2003) Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol. Aging 24, 853–860.
Bresnahan J. C. 1978. An electron microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J. Neurol. Sci. 37, 59–82.
Buck C. R., Seburn K. L., and Cope T. C. (2000) Neurotrophin expression by spinal motoneurons in adult and developing rats. J. Comp. Neurol. 416, 309–318.
Bunge R. P., Puckett, W. R., Becerra, J. L., Marcillo, A., and Quencer, R. M. (1993) Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89.
Carrol W. M., Jennings, A. R., and Ironside L. J. (1998). Identification of the adult resting progenitor cell by autoradiographic tracking of oligodendrocyte precursors in experimental CNS demyelination. Brain 121, 293–302.
Chan J. R., Cosgaya J. M., Wu Y. J., and Shooter E. M. (2001) Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc. Natl. Acad. Sci. U. S. A. 98, 14661–14668.
Ciriza I., Azcoitia I., and Garcia-Segura L. M. (2004) Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol. 16, 58–63.
Davies A. M. (1996) Paracrine and autocrine actions of neurotrophic factors. Neurochem. Res. 21, 749–753.
De Nicola A. F., Labombarda F., González S. L., González Deniselle M. C., Guennoun R., and Schumacher M. (2003) Steroid effects on glial cells: detrimental or protective for spinal cord function. Ann. N. Y. Acad. Sci. 1007, 317–328.
Desarnaud F., Do Thai A. N., Brown A. M., et al. (1998). Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J. Neurochem. 71, 1765–1768.
Dougherty K. D., Dreyfus C., and Black I. B. (2000). Brain-derived neurotrophic factor in astrocytes, oligoden-drocytes, microglia/macrophages after spinal cord injury. Neurobiol. Dis. 7, 574–585.
Dreyfus C. F., Dai X., Lercher L. D., Racey B. R., Friedman W. J., and Black I. B. (1999) Expression of neurotrophins in the adult spinal cord in vivo. J. Neurosci. Res. 56, 1–7.
Du Y., Fischer T. Z., Lee L. N., Lercher L. D., and Dreyfus C. F. (2003) Regionally specificeffects of BDNF on oligodendrocytes. Dev. Neurosci. 25, 116–128.
Eidelberg E., Nguyen L. H., Polich R., and Walden J. G. (1989) Transsynaptic degeneration of motoneurones caudal to spinal lesions. Brain Res. Bull. 22, 39–45.
Ernfors P., Kucera J., Lee K. F., Loring J., and Jaenisch R. (1995) Studies on the physiological role of brain derived neurotrophic factor and neurotrophin-3 in knockout mice. Int. J. Dev. Biol. 39, 799–807.
Falkenstein E., Heck M., Gerdes D., et al. (1999) Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology 140(12), 5999–6002.
Falkenstein E., Meyer C., Eisen, C., Scriba, P. C., and Wehling M. (1996) Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys Res. Commun. 229, 86–89.
Finkbeiner S., Tavazole, S. F., Maloratsky A., Jacobs K. M., Harris K. M., and Greenberg M. E. (1997) CREB, a major mediator of neuronal neurotrophic response. Neuron 19, 1031–1047.
Forger N., Wagner C. H., Contois M., et al. (1998) Ciliary neurotrophic factor receptor in spinal motoneurones is regulated by gonadal hormones. J. Neurosci. 18, 8720–8729.
Gerdes D., Wehling M., Leube B., and Fankelstein E. (1998) Cloning and tissue expression of two putative steroid membrane receptors. J. Biol. Chem. 379, 907–911.
Ghoumari A. M., Bauliu E. E., and Schumacher M. (2005) Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 135, 47–58.
Ghoumari A. M., Ibanez C., El-Etr M., Leclerc P., Eychenne B., O'Malley B. W., et al. (2003) Progesterone and its metabolites increase myelin basic protein expression in organotypic slices cultures of rat cerebellum. J. Neurochem. 86, 848–859.
Giehl K. M., Schutte A., Mestres P., and Yan Q. (1998) The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J. Neurosci. 18, 7351–7360.
Gomez-Pinilla F., Ying Z., Roy, R. R., Molteni R., and Edgerton V. R. (2002) Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 88, 2187–2195.
González S. L., Labombarda F., González Deniselle M. C., Guennoun R., Schumacher M., and De Nicola A. F. (2004) Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience 125, 605–614.
González Deniselle M. C., López-Costa J. J., González S. L., Labombarda F., Garay L., Guennoun R., et al. (2003) Basis of progesterone neuroprotection in spinal cord neurodegeneration. J. Steroid Biochem. Mol. Biol. 83, 199–209.
González Deniselle M. C., López-Costa J. J., Pecci Saavedra J., Pietranera L., González S. L., Garay L., et al. (2002) Progesterone neuroprotection in the wobbler mouse, a genetic model of spinal cord motor neuron disease. Neurobiol. Dis. 11, 457–468.
Grossman S. D., Rosenberg L. J., and Wrathall J. R. (2001) Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp. Neurol. 168, 273–282.
Guennoun R., Benmessahel Y., Delespierre B., Gouezou M., Rajkowski K. M., Baulieu E. E., and Schumacher M. (2001) Progesterone stimulates Krox-20 gene expression in Schwann cells. Mol. Brain Res. 90, 75–82.
Hamano K., Iwasaki N., Tayeka T., and Takita H. (1996) A quantitative analysis of rat central nervous system myelination using the immunohistochemical method for MBP. Brain Res. Dev. Brain Res. 93, 18–22.
Hansson A. C., Cintra A., Belluardo N., Sommer W., Bhatnagar M., Bader M., et al. (2000) Gluco- and mineralo-corticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and neocortex of the rat. Eur. J. Neurosci. 12, 2918–2934.
Hubbard P. (2003) The response of novel NG2 glia to spinal cord injury. Spinal Res. 1, 100–107.
Ianova T., Kuppers E., Engele J., and Beyer C. (2001) Estrogen stimulates brain-derived neurotrophic factor expression in embryonic mouse midbrain neurons through a membrane-mediated and calcium-dependent mechanism. J. Neurosci. Res. 66, 221–230.
Ibanez C., Shields S. A., Liere P., El-Etr M., Baulieu E. E., Schumacher M., and Franklin R. J. M. (2004), Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol. Appl. Neurobiol. 30, 80–89.
Ikeda O., Murakami M., Ino H., Yamazaki M., Koda M., Nakayama C., and Moriya H. (2002) Effects of brainderived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J. Neuropathol. Exp. Neurol. 61, 142–153.
Ishii K., Toda M., Nakai, Y., Asou H., Watanabe M., Nakamura, M., et al. (2001) Increase of oligodendrocyte progenitor cells after spinal cord injury. J. Neurosci. Res. 65, 500–507.
Jakeman L. B., Wei P., Guan, Z., and Stokes B. T. (1998) Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 154, 170–184.
Jung-Testas I., Do Thi A., Koenig H., Desarnaud F., Shazand K., Schumacher, M., and Baulieu E. E. (1999) Progesterone as a neurosteroid: synthesis and actions in rat glial cells. J. Steroid Biochem. Mol. Biol. 69, 97–107.
Jung-Testas I., Schumacher M., Robel P., and Baulieu E. E. (1996) The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell. Mol. Neurobiol. 16, 439–443.
Keirstead H. S. and Blackemore W. F. (1997) Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 56, 1191–1201.
Kobayashi N. R., Fan D. P., Ghiel K. M., Bedard A. M., Wiegand S. J., and Tetzlaff W. (1997) BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha 1-tubulin mRNA expression, and promote axonal regeneration. J. Neurosci. 17, 9583–9595.
Koenig H. L., Schumacher M., Ferzaz B., et al. (1995) Progesterone synthesis and myelin formation by Schwann cells. Science 268, 1500–1502.
Krebs C. J., Jarvis E. D., Chan J., Lydon J. P., Ogawa S., and Pfaff D. W. (2000) A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc. Natl. Acad. Sci. U.S.A. 97, 12816–12821.
Labombarda F., González S. L., Gonzalez Deniselle M. C., Guennoun R., Schumacher M., and De Nicola A. F. (2002) Cellular basis for progesterone neuroprotection in the injured spinal cord. J. Neurotrauma 19, 343–355.
Labombarda F., González S. L., Gonzalez Deniselle M. C., Vinson G. P., Schumacher M., De Nicola A. F., and Guennoun R. (2003) Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-DX expression in the rat spinal cord. J. Neurochem. 87, 902–913.
Labombarda F., González S. L., Roig P., et al. (2000a) Modulation of NADPH-diaphorase and glial fibrillary acidic protein by progesterone in astrocytes from normal and injured rat spinal cord. J. Steroid Biochem. Mol. Biol. 73, 159–169.
Labombarda F., Guennoun R., González S. L., et al. (2000b) Immunocytochemical evidence for a progesterone receptor in neurones and glial cells of the rat spinal cord. Neurosci. Lett. 288, 29–32.
Levine J. M., Reynolds R., and Fawcett J. W. (2001) The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 24, 39–47.
Li, G. and Blakemore W. F. (2004) The number of cells expressing the myelin-supporting oligodendrocyte marker PLP-exon 3b remains unchanged in wallerian degeneration. J. Neurotrauma 21, 1044–1049.
Magnaghi V., Cavarretta I., Galbiati M., Martini L., and Melcangi R. C. (2001) Neuroactive steroids and peripheral myelin proteins. Brain Res. Rev. 37, 360–371.
Maisonpierre P. C., Le Beau M. M., Espinosa R. III, Ip N. Y., Belluscio L., de la Monte S. M., et al. (1991) Human and rat brain-derived neurotrophic factor and neurotrophin-3 gene structures, distributions, and chromosomal localizations. Genomics 10, 558–568.
Majewska M. D., Harrison N. L., Shwartz R. D., Barker J. L., and Paul S. M. (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007.
Mattson, M. P., Maudsley S., and Martin B. (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 27, 589–594.
McTigue D. M., Horner, P. J., Stokes B. T., and Gage F. M. (1998) Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J. Neurosci. 18, 5354–5365.
Miranda R. C., Sohrabji F., and Toran-Allerand C. D. (1993) Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc. Natl. Acad. Sci. U. S. A. 90, 6439–6443.
Monks D. A., Arciszewska G., and Watson N. V. (2001) Estrogen-inducible progesterone receptors in the rat lumbar spinal cord: regulation by ovarian steroids and fluctuation across the estrous cycle. Horm. Behav. 40, 490–496.
Muse E. D., Jurevics H., Toews A. D., Matsushima G. K., and Morell P. (2001) Parameters related to lipid metabolism as markers of myelination in the mouse brain. J. Neurochem. 76, 77–86.
Nacimiento W., Sappok T., Brook G. A., Tóth L., Schoen S. W., Noth J., and Kreutzberg G. W. (1995) Structural changes of anterior horn neurons and their synaptic input caudal to low thoracic spinal cord hemisection in the adult rat: a light and electron microscopic study Acta Neuropathol. 90, 552–564.
Nakamura M. and Bregman B. S. (2001) Differences in neurotrophic factor gene expression profiles between neonate and adult spinal cord after injury. Exp. Neurol. 169, 407–415.
Nishiyama A., Chang A., and Trapp B. D. (1999) NG2+glial cells: a novel glial cell population in the adult brain. J. Neuropathol. Exp. Neurol. 58, 1113–1124.
Raza F. S., Takemori H., Tojo H. Okamoto M., and Vinson, G. P. (2001) Identification of the rat adrenal zona fasciculate/reticularis specific protein, inner zone antigen (IZAg), as the putative membrane progesterone receptor. Eur. J. Biochem. 268, 2141–2147.
Roof R. and Hall E. (2000) Gender differences in acute CNS trauma and stroke: neuro protective effects of progesterone. J. Neurotrauma 17, 367–388.
Roof R., Duvdevani R., Braswell L., et al. (1994) Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 129, 64–69.
Runko E., Wideman C., and Kaprielian Z. (1999) Cloning and expression of VEMA: a novel ventral midline antigen in the rat CNS. Mol. Cell. Neurosci. 14, 428–443.
Rupprecht R., Hauser C. A. E., Trapp T., and Holsboer F. (1996) Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J. Steroid Biochem. Mol. Biol. 56, 163–168.
Sayer F. T., Oudega M., and Hagg T. (2002) Neurotrophins reduce degeneration of injured ascending sensory and corticospinal motor axons in adult rat spinal cord. Exp. Neurol. 175, 282–296.
Schober A., Wolf N., Kahane N., Kalcheim C., Krieglstein K., and Unsicker K. (1999) Expression of neurotrophin receptors trkB and trkC and their ligands in rat adrenal gland and the intermediolateral column of the spinal cord. Cell Tissue Res. 296, 271–279.
Schumacher M., Akwal I., Guennoun R., robert F., Labombarda F., Desarnaud P., et al. (2000) Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J. Neurocytol. 29, 307–326.
Selmin O., Lucier G. W., Clark G. C., Tritscher A. M., Vanden Heuvel J. P., Gastel J. A., et al. (1996) Isolation and characterization of a nivel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesis 17, 2609–2615.
Sim F. J., Hinks G. L., and Franklin R. J. (2000) The re-expression of the homeodomain transcription factor Gtx during remyelination of experimentally induced demyelinating lesions in young and old rat brain. Neuroscience 100, 131–139.
Skup M., Dwornik A., Macias M., Suleiczak D., Wiater M., and Czarkowska-Bauch J. (2002) Long-term locomotor training up-regulates trkBFL receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurones in the spinal cord. Exp. Neurol. 176, 289–307.
Sohrabji F., Miranda R. C., and Toran-Allerand C. D. (1995) Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U. S. A. 92, 11110–11114.
Solum D. T. and Handa R. J. (2002) Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 22, 2650–2659.
Stein D. G. (2001) Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24, 386–391.
Stein D. G. and Fulop Z. L. (1998) Progesterone and recovery after traumatic brain injury: an overview. Neuroscientist 4, 435–442.
Tanridag T., Coskun T. Hurdag C., Arbak S., Aktan S., and Yegen B. (1999) Motor neuron degeneration due to aluminium depositon in the spinal cord: a light microscopical study. Acta Histochem. 101, 193–201.
Thoenen H. (1995) Neurotrophins and neuronal plasticity. Science 270, 593–598.
Thomas A., Nockels R., Pan H., et al. (1999) Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine 24, 2134–2138.
Tolwani R. J., Cosyaga J. M., Varma S., Jacob R., Kuo L. E., and Shooter E. M. (2004) BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J. Neurosci. Res. 77, 662–669.
Verdi J. M. and Campagnoni A. T. (1990) Translational regulation by steroids. Identification of a steroid modulatory element in the 5′-untranslated region of the myelin basic protein messenger RNA. J. Biol. Chem. 265, 20314–20320.
Wakayama I. (1992) Morphometry of spinal motor neurons in amyotrophic lateral sclerosis with special reference to chromatolysis and intracytoplasmic inclusion bodies. Brain Res. 586, 12–18.
Walton M. R. and Dragunow M. (2000) Is CREB a key to neuronal survival? Trends Neurosci. 23, 48–53.
Winkler T., Sharma H. S., Stalberg E., and Badgaiyan R. D. (2000) Neurotrophic factors attenuate alterations in spinal cord evoked potentials and edema formation following trauma to the rat spinal cord. Acta Neurochir. Suppl. 76, 291–296.
Yan Q., Matheson C., Lopez O., et al. (1994) The biological responses of axotomized adult motoneurones to brain-derived neurotrophic factor. J. Neurosci. 14, 5281–5291.
Ye P., Bagnell R., and D'Ercole A. J. (2003) Mouse NG2 + oligodendrocyte precursors express mRNA for proteolipid protein but not its DM-20 variant: a study of laser microdissection-captured NG2 + cells. J. Neurosci. 23, 4401–4405.
Young I. J. (1966) Morphological and histochemical studies of partially and totally deafferented spinal cord segments. Exp. Neurol. 14, 238–248.
Yu W. H. (1989) Survival of motoneurones following axotomy is enhanced by lactation or by progesterone treatment. Brain Res. 49, 379–382.
Zhang J.-Y., Luo X.-G., Xian C. J., Liu Z.-H., and Zhou X.-F. (2000) Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur. J. Neurosci. 12, 4171–4180.
Zhu, Y., Bond J., and Thomas P. (2003) Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. U. S. A. 100, 2237–2242.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Nicola, A.F., Gonzalez, S.L., Labombarda, F. et al. Progesterone treatment of spinal cord injury. J Mol Neurosci 28, 3–15 (2006). https://doi.org/10.1385/JMN:28:1:3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:28:1:3