Abstract
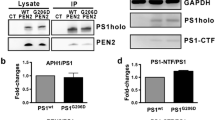
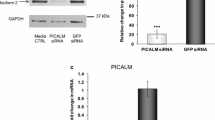
The γ-secretase complex consists of PS1/PS2, nicastrin, APH-1a, and PEN-2. PS1 undergoes endoproteolytic processing to yield two fragments: PS1-NTF and PS1-CTF. Changes in PEN-2 levels have been shown previously to affect the endoproteolytic processing of wild-type (wt)-PS1. However, the effects of PEN-2 on the proteolytic processing of familial Alzheimer’s disease (FAD) mutant forms of PS1 have not yet been reported. To determine whether PEN-2 affects the proteolytic processing of mutant PS1 in the same manner as that of wt-PS1, we established RNA interference (RNAi) for PEN-2 in H4 human neuroglioma cells stably transfected to express wt or FAD mutant forms of PS1 including L286V, A246E, and that lacking exon 9 (Δ9). As expected, in H4 cells expressing wt-PS1, RNAi for PEN-2 increased levels of PS1-FL and attenuated PS1 endoproteolysis. Likewise, in cells expressing PS1 with the FAD missense mutations, L286V and A246E, RNAi for PEN-2 increased PS1-FL and reduced PS1 endoproteolysis. However, in H4 cells stably transfected to express the FAD-linked Δ9 mutation (PS1 lacking exon 9), RNAi for PEN-2 did not increase but, instead, decreased PS1-FL. In contrast, RNAi for nicastrin and APH-1a decreased PS1-FL in H4 cells expressing either wt-PS1 or Δ9-PS1. In summary, the metabolism of wt-PS1 and FAD-linked Δ9-PS1 is specifically and differentially affected by loss of function of PEN-2.
Similar content being viewed by others
References
De Strooper B. (2003) Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron 38, 9–12.
De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., Cordell B., et al. (1997) Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J. Biol. Chem. 272, 3590–3598.
Doan A., Thinakaran G., Borchelt D. R., Slunt H. H., Ratovitsky T., Podlisny M., et al. (1996) Protein topology of presenilin 1. Neuron 17, 1023–1030.
Edbauer D., Winkler E., Haass C., and Steiner H. (2002) Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc. Natl. Acad. Sci. USA 99, 8666–8671.
Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., and Haass C. (2003) Reconstitution of gamma-secretase activity. Nat. Cell Biol. 5, 486–488.
Fraering P. C., LaVoie M. J., Ye W., Ostaszewski B. L., Kimberly W. T., Selkoe D. J., and Wolfe M. S. (2004) Detergent-dependent dissociation of active gamma-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry 43, 323–333.
Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., Apfeld J., et al. (2002) aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of beta APP, and presenilin protein accumulation. Dev. Cell 3, 85–97.
Gu Y., Chen F., Sanjo N., Kawarai T., Hasegawa H., Duthie M., et al. (2003) APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin.nicastrin complexes. J. Biol. Chem. 278, 7374–7380.
Kim S. H., Ikeuchi T., Yu C. and Sisodia S. S. (2003) Regulated hyperaccumulation of presenilin-1 and the “gamma-secretase” complex. Evidence for differential intramembranous processing of transmembrane subatrates. J. Biol. Chem. 278, 33,992–34,002.
Kim T. W., Pettingell W. H., Jung Y. K., Kovacs D. M., and Tanzi R. E. (1997) Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277, 373–376.
Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., and Selkoe D. J. (2003a) Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. USA 100, 6382–6387.
Kimberly W. T., Esler W. P., Ye W., Ostaszewski B. L., Gao J., Diehl T., et al. (2003b) Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s). Biochemistry 42, 137–144.
Kovacs D. M., Mancini R., Henderson J., Na S. J., Schmidt S. D., Kim T. W., and Tanzi R. E. (1999) Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer’s disease mutations in human neuroglioma cells. J. Neurochem. 73, 2278–2285.
LaVoie M. J., Fraering P. C., Ostaszewski B. L., Ye W., Kimberly W. T., Wolfe M. S., and Selkoe D. J. (2003) Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J. Biol. Chem. 278, 37,213–37,222.
Lee S. F., Shah S., Li H., Yu C., Han W., and Yu G. (2002) Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J Biol Chem 277, 45,013–45,019.
Li X. and Greenwald I. (1996) Membrane topology of the C. elegans SEL-12 presenilin. Neuron 17, 1015–1021.
Li Y. M., Lai M. T., Xu M., Huang Q., DiMuzio-Mower J., Sardana M. K., et al. (2000) Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc. Natl. Acad. Sci. USA 97, 6138–6143.
Luo W. J., Wang H., Li H., Kim B. S., Shah S., Lee H. J., et al. (2003) PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J. Biol. Chem. 278, 7850–7854.
Morais V. A., Crystal A. S., Pijak D. S., Carlin D., Costa J., Lee V. M., and Doms R. W. (2003) The transmembrane domain region of nicastrin mediates direct interactions with APH-1 and the gamma-secretase complex. J. Biol. Chem. 278, 43,284–43,291.
Nyabi O., Bentahir M., Horre K., Herreman A., Gottardi-Littell N., Van Broeckhoven C., et al. (2003) Presenilins mutated at Asp-257 or Asp-385 restore Pen-2 expression and Nicastrin glycosylation but remain catalytically inactive in the absence of wild type Presenilin. J. Biol. Chem. 278, 43,430–43,436.
Perez-Tur J., Froelich S., Prihar G., Crook R., Baker M., Duff K., et al. (1995) A mutation in Alzheimer’s disease destroying a splice acceptor site in the presenilin-1 gene. Neuroreport 7, 297–301.
Prokop S., Shirotani K., Edbauer D., Haass C., and Steiner H. (2004) Requirement of PEN-2 for Stabilization of the Presenilin N-/C-terminal Fragment Heterodimer within the γ-Secretase Complex. J. Biol. Chem. 279, 23,255–23,261.
Ratovitski T., Slunt H. H., Thinakaran G., Price D. L., Sisodia S. S., and Borchelt D. R. (1997) Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J. Biol. Chem. 272, 24,536–24,541.
Shirotani K., Edbauer D., Capell A., Schmitz J., Steiner H., and Haass C. (2003) Gamma-secretase activity is associated with a conformational change of nicastrin. J. Biol. Chem. 278, 16,474–16,477.
Steiner H., Winkler E., Edbauer D., Prokop S., Basset G., Yamasaki A., et al. (2002) PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J. Biol. Chem. 277, 39,062–39,065.
Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., et al. (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422, 438–441.
Tanzi R. E. and Bertram L. (2001) New frontiers in Alzheimer’s disease genetics. Neuron 32, 181–184.
Thinakaran G. (1999) The role of presenilins in Alzheimer’s disease. J. Clin. Invest. 104, 1321–1327.
Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190.
Xie Z., Moir, R., Romano, D. M., Tesco, G., Kovacs, D., and Tanzi, R. (2004) Hypocapnia Induces Caspase-3 Activation and Increases A-beta Production. Neurodegenerative Disease 1, 29–37.
Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., et al. (2000) Nicastrin modulates presenilinmediated notch/glp-1 signal transduction and beta APP processing. Nature 407, 48–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Z., Romano, D.M. & Tanzi, R.E. Effects of RNAi-mediated silencing of PEN-2, APH-1a, and nicastrin on wild-type vs FAD mutant forms of presenilin 1. J Mol Neurosci 25, 67–77 (2005). https://doi.org/10.1385/JMN:25:1:067
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:25:1:067