Abstract
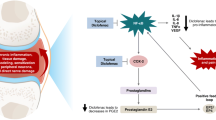
Endomorphin-1 is a selective endogenous ligand for the μ-opioid receptor, and this study investigated the effect of endomorphin-1 on rat knee joint inflammation by examining the ability of the neuropeptide to modulate synovial protein extravasation. Acute joint inflammation was induced by intraarticular injection of 2% kaolin followed by 2% carrageenan and the animals allowed to recover for 3 h. Immunohistochemical examination of these inflamed joints revealed endomorphin-1-like immunoreactive nerves in deep synovium with a proportion of the nerve fibers occurring in close proximity to synovial blood vessels. Perfusion of inflamed knees with exogenous endomorphin-1 across the dose range 10−9−10−6 M produced a significant reduction in synovial vascular permeability with the 10−7 M dose producing the greatest fall in protein exudation (approx 55%). These effects were blocked by the specific μ-opioid receptor antagonist CTOP. Destruction of knee joint unmyelinated afferent nerve fibers by capsaicin treatment significantly attenuated the anti-inflammatory effects of endomorphin-1, suggesting that the peptide is acting via a neurogenic mechanism. The findings of this study indicate that endomorphin-1 acts peripherally in knee joints to reduce synovial protein extravasation. These anti-inflammatory effects are mediated by μ-opioid receptors located on capsaicin-sensitive afferent nerves.
Similar content being viewed by others
References
Averbeck B., Reeh P. W., and Michaelis M. (2001) Modulation of CGRP and PGE2 release from isolated rat skin by alpha-adrenoceptors and kappa-opioid-receptors. Neuroreport. 12, 2097–2100.
Binder W. and Walker J. S. (1998) Effect of the peripherally selective kappa-opioid agonist, asimadoline, on adjuvant arthritis. Br. J. Pharmacol. 124, 647–654.
Brodin E., Gazelius B., Panopoulos P., and Olgart L. (1983) Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol. Scand. 117, 567–570.
Chao C. C., Molitor T. W., Close K., Hu S., and Peterson P. K. (1993) Morphine inhibits the release of tumor necrosis factor in human peripheral blood mononuclear cell cultures. Int. J. Immunopharmacol. 15, 447–453.
Coggeshall R. E., Zhou S., and Carlton S. M. (1997) Opioid receptors on peripheral sensory axons. Brain Res. 764, 126–132.
Colpaert F. C., Donnerer J., and Lembeck F. (1983) Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 32, 1827–1834.
Courtright L. J. and Kuzell W. C. (1965) Sparing effect of neurological deficit and trauma on the course of adjuvant arthritis in the rat. Ann. Rheum. Dis. 24, 360–368.
Cruwys S. C., Garrett N. E., and Kidd B. L. (1995) Sensory denervation with capsaicin attenuates inflammation and nociception in arthritic rats. Neurosci. Lett. 193, 205–207.
Del Bianco E., Maggi C. A., Santicioli P., and Geppetti P. (1994) Modulation of calcitonin gene-related peptide release evoked by bradykinin and electrical field stimulation in guinea-pig atria. Neurosci. Lett. 170, 163–166.
Di Rosa M. (1972) Biological properties of carrageenan. J. Pharm. Pharmacol. 24, 89–102.
Dun N. J., Dun S. L., Wu S. Y., Williams C. A., and Kwok E. H. (2000) Endomorphins: localization, release and action on rat dorsal horn neurons. J. Biomed. Sci. 7, 213–220.
Ferrell W. R. and Russell N. J. W. (1986) Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetised cat. J. Physiol. 379, 407–416.
Ferrell W. R., Lam F., and Montgomery I. (1992) Differences in the axon composition of nerves supplying the rat knee joint following intra-articular injection of capsaicin. Neurosci. Lett. 141, 259–261.
Gardner D. L. (1960) Production of arthritis in the rabbit by the local injection of the mucopolysaccharide carragheenin. Ann. Rheum. Dis. 19, 369–376.
Green P. G. and Levine J. D. (1992) Delta- and kappa-opioid agonists inhibit plasma extravasation induced by bradykinin in the knee joint of the rat. Neuroscience. 49, 129–133.
Hackler L., Zadina J. E., Ge L. J., and Kastin A. J. (1997) Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 18, 1635–1639.
Iwata J. and Nishikaze O. (1979) New micro-turbidimetric method for determination of protein in cerebrospinal fluid and urine. Clin. Chem. 25, 1317–1319.
Jessop D. S., Richards L. J., and Harbuz M. S. (2002) Opioid peptides endomorphin-1 and endomorphin-2 in the immune system in humans and in a rodent model of inflammation. Ann. NY Acad. Sci. 966, 456–463.
Ji R. R., Zhang Q., Law P. Y., Low H. H., Elde R., and Hokfelt T. (1995) Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J. Neurosci. 15, 8156–8166.
Joshi G. P., McCarroll S. M., McSwiney M., O’Rourke P., and Hurson B. J. (1993) Effects of intraarticular morphine on analgesic requirements after anterior cruciate ligament repair. Reg. Anesth. 18, 254–257.
Karimian S. M. and Ferrell W. R. (1994) Plasma protein extravasation into the rat knee joint induced by calcitonin gene-related peptide. Neurosci. Lett. 166, 39–42.
Khalil Z., Sanderson K., Modig M., and Nyberg F. (1999) Modulation of peripheral inflammation by locally administered endomorphin-1. Inflamm. Res. 48, 550–556.
Lam F. Y. and Ferrell W. R. (1990) Mediators of substance-P-induced inflammation in the rat knee joint. Agents Actions. 31, 298–307.
Lam F. Y. and Ferrell W. R. (1991) Specific neurokinin receptors mediate plasma extravasation in the rat knee joint. Br. J. Pharmacol. 103, 1263–1267.
Levine J. D., Moskowitz M. A., and Basbaum A. I. (1985) The contribution of neurogenic inflammation in experimental arthritis. J. Immunol. 135, 843s-847s.
Levine J. D., Dardick S. J., Roizen M. F., Helms C., and Basbaum A. I. (1986) Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J. Neurosci. 6, 3423–3429.
Likar R., Schafer M., Paulak F., Sittl R., Pipam W., Schalk H., et al. (1997) Intraarticular morphine analgesia in chronic pain patients with osteoarthritis. Anaesth. Analg. 84, 1313–1317.
Martin-Schild S., Gerall A. A., Kastin A. J., and Zadina J. E. (1998) Endomorphin-2 is an endogenous opioid in primary sensory afferent fibers. Peptides 19, 1783–1789.
Martin-Schild S., Gerall A. A., Kastin A. J., and Zadina J. E. (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol. 405, 450–471.
Martin-Schild S., Zadina J. E., Gerall A. A., Vigh S., and Kastin A. J. (1997) Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides 18, 1641–1649.
Ninkovic M., Hunt S. P., and Gleave J. R. (1982) Localization of opiate and histamine H1-receptors in the primate sensory ganglia and spinal cord. Brain Res. 241, 197–206.
Peterson P. K., Sharp B., Gekker G., Brummitt C., and Keane W. F. (1987) Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J. Clin. Invest. 80, 824–831.
Schreff M., Schulz S., Wiborny D., and Hollt V. (1998) Immunofluorescent identification of endomorphin-2-containing nerve fibers and terminals in the rat brain and spinal cord. Neuroreport. 9, 1031–1034.
Scott D. T., Lam F. Y., and Ferrell W. R. (1991) Time course of substance P-induced protein extravasation in the rat knee joint measured by micro-turbidimetry. Neurosci. Lett. 129, 74–76.
Scott D. T., Lam F. Y., and Ferrell W. R. (1992) Acute inflammation enhances substance P-induced plasma protein extravasation in the rat knee joint. Reg. Pep. 39, 227–235.
Sharp B. M., Roy S., and Bidlack J. M. (1998) Evidence for opioid receptors on cells involved in host defense and the immune system. J. Neuroimmunol. 83, 45–56.
Sheehy J. G., Hellyer P. W., Sammonds G. E., Mama K. R., Powers B. E., Hendrickson D. A., et al. (2001) Evaluation of opioid receptors in synovial membranes of horses. Am. J. Vet. Res. 62, 1408–1412.
Stein C., Hassan A. H., Przewlocki R., Gramsch C., Peter K., and Herz A. (1990) Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc. Natl. Acad. Sci. USA 87, 5935–5939.
Stein C., Comisel K., Haimerl E., Yassouridis A., Lehrberger K., Herz A., et al. (1991) Analgesic effect of intraarticular morphine after arthroscopic knee surgery. New Engl. J. Med. 325, 1123–1126.
Walker J. S., Chandler A. K., Wilson J. L., Binder W., and D R. O. (1996) Effect of mu-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm. Res. 45, 557–563.
Wilson J. L., Nayanar V., and Walker J. S. (1996) The site of anti-arthritic action of the kappa-opioid, U-50, 488H, in adjuvant arthritis: importance of local administration. Br. J. Pharmacol. 118, 1754–1760.
Yaksh T. L. (1988) Substance P release from knee joint afferent terminals; modulation by opioids. Brain Res. 458, 319–324.
Zadina J. E., Hackler L., Ge L. J., and Kastin A. J. (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386, 499–502.
Zadina J. E., Martin-Schild S., Gerall A. A., Kastin A. J., Hackler L., Ge L. J., et al. (1999) Endomorphins: novel endogenous mu-opiate receptor agonists in regions of high mu-opiate receptor density. Ann. NY Acad. Sci. 897, 136–144.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDougall, J.J., Baker, C.L. & Hermann, P.M. Attenuation of knee joint inflammation by peripherally administered endomorphin-1. J Mol Neurosci 22, 125–137 (2004). https://doi.org/10.1385/JMN:22:1-2:125
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:22:1-2:125