Abstract
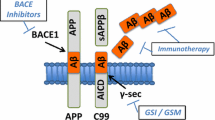
Research over the past ten years on Alzheimer’s disease has pursued many opportunities. Notable amongst the various approaches are efforts related to the “amyloid hypothesis.” This hypothesis posits that the beta amyloid peptide causes the extensive neuropathology and clinical decline associated with the disease. Extensive research in this area has shown that the beta amyloid peptide is produced by proteases termed “secretases” and it has been shown that blockade of secretase functions reduce the amount of beta amyloid peptide produced. An additional approach to reduce beta amyloid, through an increase in clearance mechanisms, is to immunize with the peptide itself and induce an antibody response. The specifically elicited antibodies then bind to and stimulate clearance of the peptide from the brain. These findings have stimulated several approaches to develop novel therapeutic strategies to treat Alzheimer’s disease that either are about or have entered the clinic.
Similar content being viewed by others
References
Bacskai B. J., Kajdasz S. T., Christie R. H., Carter C., Games D., Seubert P., et al. (2001) Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nature Med. 7, 369–372.
Bard F., Cannon C., Barbour R., Burke R. L., Games D., Grajeda H., et al. (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 6, 916–919.
Bothwell M., and Giniger E. (2000) Alzheimer’s disease: neurodevelopment converges with neurodegeneration. Cell. 102, 271–273.
Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., et al. (1991) Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature 353, 844–846.
Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., and Selkoe D. J. (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360, 672–674.
Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., et al. (1997) Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice [see comments]. Nature Med. 3, 67–72.
De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., et al. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein [see comments]. Nature 391, 387–390.
Dormanen M. C., Bishop J. E., Hammond M. W., Okamura W. H., Nemere I., and Norman A. W. (1994) Nonnuclear effects of the steroid hormone 1 alpha,25(OH) 2-vitamin D3: analogs are able to functionally differentiate between nuclear and membrane receptors. Biochem. Biophys. Res. Commun. 201, 394–401.
Dovey H. F., John V., Anderson J. P., Chen L. Z., de Saint Andrieu P., Fang L. Y., et al. (2001) Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 76, 173–181.
Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Pereztur J., et al. (1996) Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713.
Duff K., Knight H., Refolo L. M., Sanders S., Yu X., Picciano M., et al. (2000) Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol. Dis. 7, 87–98.
El-Agnaf O. M., Guthrie D. J., Walsh D. M., and Irvine G. B. (1998) The influence of the central region containing residues 19–25 on the aggregation properties and secondary structure of Alzheimer’s beta-amyloid peptide [In Process Citation]. Eur. J. Biochem. 256, 560–569.
Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., et al. (1990) Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248, 1122–1124.
Esler W. P., Kimberly W. T., Ostaszewski B. L., Diehl T. S., Moore C. L., Tsai J. Y., et al. (2000) Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nature Cell Biol. 2, 428–434.
Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., et al. (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein [see comments]. Nature 373, 523–527.
Giacobini E. (2000) Cholinesterase inhibitors stabilize Alzheimer disease. Neurochem. Res. 25, 1185–1190.
Glenner G. G., and Wong C. W. (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890.
Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease [see comments]. Nature 349, 704–706.
Grundman M., and Thal L. J. (2000) Treatment of Alzheimer’s disease: rationale and strategies. Neurol. Clin. 18, 807–828.
Grutzendler J., and Morris J. C. (2001) Cholinesterase inhibitors for Alzheimer’s disease. Drugs 61, 41–52.
Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., et al. (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism [see comments]. Nature 359, 322–325.
Hardy J. (1996) New insights into the genetics of Alzheimer’s disease. Ann. Med. 28, 255–258.
Hardy J., Chartier-Harlin M. C., and Mullan M. (1992) Alzheimer disease: the new agenda. Am. J. Human Genet. 50, 648–651.
Higaki J., Quon D., Zhong Z., and Cordell B. (1995) Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron 14, 651–659.
Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., et al. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice [see comments]. Science 274, 99–102.
Huang X., Atwood C. S., Moir R. D., Hartshorn M. A., Vonsattel J. P., Tanzi R. E., and Bush A. I. (1997) Zincinduced Alzheimer’s Abeta 1–40 aggregation is mediated by conformational factors. J. Biol. Chem. 272, 26,464–26,470.
Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., and Ihara Y. (1994) Visualization of A beta 42(43) and A beta 40 in senile plaques with end- specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13, 45–53.
Janus C., Pearson J., McLaurin J., Mathews P. M., Jiang Y., Schmidt S. D., et al. (2000) A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408, 979–982.
Kopan R., and Goate A. (2000) A common enzyme connects notch signaling and Alzheimer’s disease. Genes Dev. 14, 2799–2806.
Kulic L., Walter J., Multhaup G., Teplow D. B., Baumeister R., Romig H., Capell A., Steiner H., and Haass C. (2000) Separation of presenilin function in amyloid beta-peptide generation and endoproteolysis of Notch. Proc. Natl. Acad. Sci. USA 97, 5913–5918.
Lemere C. A., Lopera F., Kosik K. S., Lendon C. L., Ossa J., Saido T. C., et al. (1996) The E280A presenilin 1 Alzheimer mutation produces increased Abeta 42 deposition and severe cerebellar pathology. Nature Med. 2, 1146–1150.
Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., DiMuzio-Mower J., et al. (2000) Photoactivated gammasecretase inhibitors directed to the active site covalently label presenilin 1. Nature 405, 689–694.
Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., et al. (2001) Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nature Neuroscience 4, 231–232.
Masliah E., Sisk A., Mallory M., Mucke L., Schenk D., and Games D. (1996) Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer’s disease. J. Neurosci. 16, 5795–5811.
Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., et al. (2000) A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 408, 982–985.
Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., and Lannfelt L. (1992a) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat. Genet. 1, 345–347.
Mullan M., Houlden H., Windelspecht M., Fidani L., Lombardi C., Diaz P., et al. (1992b) A locus for familial early-onset Alzheimer’s disease on the long arm of chromosome 14, proximal to the alpha 1-antichymotrypsin gene. Nat. Genet. 2, 340–342.
Naslund J., Haroutunian V., Mohs R., Davis K. L., Davies P., Greengard P., and Buxbaum J. D. (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline [see comments]. JAMA 283, 1571–1577.
Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., and Kokmen E. (1999) Mild cognitive impairment: clinical characterization and outcome [published erratum appears in Arch Neurol 1999 Jun;56(6):760]. Arch. Neurol. 56, 303–308.
Petit A., Bihel F., da Costa C. A., Pourquie O., Checler F., and Kraus J. L. (2001) New protease inhibitors prevent gamma-secretase-mediated production of Abeta40/42 without affecting Notch cleavage. Nat. Cell Biol. 3, 507–511.
Roberds S. L., Anderson J., Basi G., Bienkowski M. J., Branstetter D. G., Chen K. S., et al. (2001). BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Human Mol. Genet. 10, 1317–1324.
Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., et al. (1999) Immunization with amyloidbeta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [see comments]. Nature 400, 173–177.
Schenk D. B., P Seubert., Lieberburg I., and Wallace J. (2000) beta-peptide immunization: a possible new treatment for Alzheimer disease. Arch. Neurol. 57, 934–936.
Selkoe D. J. (1993) Physiological production of the beta-amyloid protein and the mechanism of Alzheimer’s disease. Trends Neurosci. 16, 403–409.
Selkoe D. J. (2000) The genetics and molecular pathology of Alzheimer’s disease: roles of amyloid and the presenilins. Neurol. Clin. 18, 903–922.
Seubert P., Oltersdorf T., Lee M. G., Barbour R., Blomquist C., Davis D. L., et al. (1993) Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 361, 260–263.
Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., et al. (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359, 325–327.
Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., et al. (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain [see comments]. Nature 402, 537–540.
Sisodia S. S. (1992) Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc. Natl. Acad. Sci. USA 89, 6075–6079.
Sisodia S. S., Kim S. H., and Thinakaran G. (1999) Function and dysfunction of the presenilins. [Review] [42 refs]. Am. J. Human Genet. 65, 7–12.
Solomon B., Koppel R., Hanan E., and Katzav T. (1996) Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc. Natl. Acad. Sci. USA 93, 452–455.
Sramek J. J., and Cutler N. R. (2000) Ongoing trials in Alzheimer’s disease. Expert. Opin. Investig. Drugs 9, 899–915.
Steiner H., Duff K., Capell A., Romig H., Grim M. G., Lincoln S., et al. (1999) A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J. Biol. Chem. 274, 28,669–28,673.
Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K. H., Mistl C., Rothacher S., et al. (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 94, 13,287–13,292.
Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr., Eckman C., et al. (1994) An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264, 1336–1340.
Thal L. J. (2000) Trials to slow progression and prevent disease onset. J. Neural. Transm. Suppl. 59, 243–249.
Tolnay M., and Probst A. (2001) Frontotemporal lobar degeneration. An update on clinical, pathological and genetic findings. Gerontology 47, 1–8.
Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., et al. (1999) beta-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 286, 735–741.
Wolfe M. S., De Los Angeles J., Miller D. D., Xia W., and Selkoe D. J. (1999) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. [Review] [81 refs]. Biochemistry 38, 11,223–11,230.
Xia W., Ray W. J., Ostaszewski B. L., Rahmati T., Kimberly W. T., Wolfe M. S., et al. (2000) Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid beta-proteing generation. Proc. Natl. Acad. Sci. USA 97, 9299–9304.
Yan R., Bienkowski M. J., Shuck M. E., Miao H., Tory M. C., Pauley A. M., et al. (1999) Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity [see comments]. Nature 402, 533–537.
Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and beta APP processing. Nature 407, 48–54.
Zhao J., Paganini L., Mucke L., Gordon M., Refolo L., Carman M., et al. (1996) Beta-secretase processing of the beta-amyloid precursor protein in transgenic mice is efficient in neurons but inefficient in astrocytes. J. Biol. Chem. 271, 31,407–31,411.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schenk, D., Games, D. & Seubert, P. Potential treatment opportunities for Alzheimer’s disease through inhibition of secretases and Aβ immunization. J Mol Neurosci 17, 259–267 (2001). https://doi.org/10.1385/JMN:17:2:259
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:17:2:259