Abstract
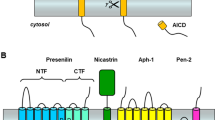
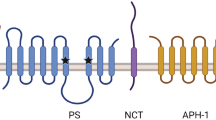
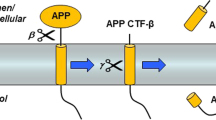
Mutations in the presenilins cause Alzheimer’s disease (AD) and alter γ-secretase activity to increase the production of the 42-residue amyloid-β peptide (Aβ) found disproportionally in the cerebral plaques that characterize the disease. The serpentine presenilins are required for transmembrane cleavage of both the amyloid-β precursor protein (APP) and the Notch receptor by γ-secretase, and presenilins are biochemically associated with the protease. Inhibitors of γ-secretase have provided critical clues to the function of presenilins. Pharmacological profiling suggested that γ-secretase is an aspartyl protease, leading to the identification of two conserved aspartates important to presenilin’s role in proteolysis. Conversion of transition-state analogue inhibitors of γ-secretase to affinity reagents resulted in specific tagging of the heterodimeric form of presenilins, strongly suggesting that the active site of γ-secretase lies at the interface of the presenilin heterodimer. Heterodimeric presenilin appears to be the catalytic portion of a multi-protein γ-secretase complex.
Similar content being viewed by others
References
Berezovska O., Jack C., McLean P., Aster J. C., Hicks C., et al. (2000) Aspartate mutations in presenilin and γ-secretase inhibitors both impair Notch1 proteolysis and nuclear translocation with relative preservation of Notch1 signaling. J. Neurochem. 75(2), 583–593.
Citron M., Diehl T. S., Gordon G., Biere A. L., Seubert P., et al. (1996) Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc. Natl. Acad. Sci. USA 93(23), 13,170–13,175.
De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., et al. (1999a) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522.
De Strooper B. and Konig G. (1999b) Alzheimer’s disease. A firm base for drug development. Nature 402(6761), 471–472.
De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., et al. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391(6665), 387–390.
Esler W. P., Kimberly W. T., Ostaszewski B. L., Diehl T. S., Moore C. L., et al. (2000) Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biol. 2(7), 428–434.
Ghosh A. K., Shin D., Downs D., Koelsch G., Lin X., et al. (2000) Design of potent inhibitors for human brain memapsin 2 (beta-secretase). J. Am. Chem. Soc. 122, 3522–3523.
Higaki J., Quon D., Zhong Z., and Cordell B. (1995) Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron 14(3), 651–659.
Hong L., Koelsch G., Lin X., Wu S., Terzyan S., et al. (2000) Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290(5489), 150–153.
Huff J. R. (1991) HIV protease: a novel chemotherapeutic target for AIDS. J. Med. Chem. 34(8), 2305–2314.
James M. N., Sielecki A. R., Hayakawa K., and Gelb M. H. (1992) Crystallographic analysis of transition state mimics bound to penicillopepsin: difluorostatine-and difluorostatone-containing peptides. Biochemistry 31(15), 3872–3886.
Klafki H., Abramowski D., Swoboda R., Paganetti P. A., and Staufenbiel M. (1996) The carboxyl termini of beta-amyloid peptides 1–40 and 1–42 are generated by distinct gamma-secretase activities. J. Biol. Chem. 271(45), 28,655–28,659.
Levitan D., Doyle T. G., Brousseau D., Lee M. K., Thinakaran G., et al. (1996) Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93(25), 14,940–14,944.
Levitan D. and Greenwald I. (1995) Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 377(6547), 351–354.
Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269(5226), 973–977.
Li Y. M., Lai M. T., Xu M., Huang Q., DiMuzio-Mower J., et al. (2000a) Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc. Natl. Acad. Sci. USA 97(11), 6138–6143.
Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., et al. (2000b) Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405(6787), 689–694.
Marciniszyn J., Jr., Hartsuck J. A., and Tang J. (1976) Mode of inhibition of acid proteases by pepstatin. J. Biol. Chem. 251(22), 7088–7094.
Moore C. L., Leatherwood D. D., Diehl T. S., Selkoe D. J., and Wolfe M. S. (2000) Difluoro Ketone Peptidomimetics Suggest a Large S1 Pocket for Alzheimer’s γ-Secretase: implications for Inhibitor Design. J. Med. Chem. 43(18), 3434–3442.
Naruse S., Thinakaran G., Luo J. J., Kusiak J. W., Tomita T., et al. (1998) Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 21(5), 1213–1221.
Ratovitski T., Slunt H. H., Thinakaran G., Price D. L., Sisodia S. S., et al. (1997) Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J. Biol. Chem. 272(39), 24,536–24,541.
Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., et al. (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376(6543), 775–778.
Schroeter E. H., Kisslinger J. A., and Kopan R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393(6683), 382–386.
Schwarzman A. L., Singh N., Tsiper M., Gregori L., Dranovsky A., et al. (1999) Endogenous presenilin 1 redistributes to the surface of lamellipodia upon adhesion of Jurkat cells to a collagen matrix. Proc. Natl. Acad. Sci. USA 96(14), 7932–7937.
Seiffert D., Bradley J. D., Rominger C. M., Rominger D. H., Yang F., et al. (2000) Presenilin-1 and -2 are molecular targets for gamma-secretase inhibitors. J. Biol. Chem. 275(44), 34,086–34,091.
Shearman M. S., Beher D., Clarke E. E., Lewis H. D., Harrison T., et al. (2000) L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry 39(30), 8698–8704.
Shen J., Bronson R. T., Chen D. F., Xia W., Selkoe D. J., et al. (1997) Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89(4), 629–639.
Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375(6534), 754–760.
Silva A. M., Cachau R. E., Sham H. L., and Erickson J. W. (1996) Inhibition and catalytic mechanism of HIV-1 aspartic protease. J. Mol. Biol. 255(2), 321–346.
Steiner H., Romig H., Pesold B., Philipp U., Baader M., et al. (1999) Amyloidogenic function of the Alzheimer’s disease-associated presenilin 1 in the absence of endoproteolysis. Biochemistry 38(44), 14,600–14,605.
Thaisrivongs S., Pals D. T., Kati W. M., Turner S. R., Thomasco L. M., et al. (1986) Design and synthesis of potent and specific renin inhibitors containing difluorostatine, difluorostatone, and related analogues. J. Med. Chem. 29(10), 2080–2087.
Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17(1), 181–190.
Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., et al. (1997) Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272(45), 28,415–28,422.
Wolfe M. S., Citron M., Diehl T. S., Xia W., Donkor I. O., et al. (1998) A substrate-based difluoro ketone selectively inhibits Alzheimer’s γ-secretase activity. J. Med. Chem. 41(1), 6–9.
Wolfe M. S., De Los Angeles J., Miller D. D., Xia W., and Selkoe D. J. (1999a) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. Biochemistry 38(35), 11,223–11,230.
Wolfe M. S., and Haass C. (2001) The role of presenilins in gamma-secretase activity. J. Biol. Chem. 276(8), 5413–5416.
Wolfe M. S., Xia W., Moore C. L., Leatherwood D. D., Ostaszewski B., et al. (1999b) Peptidomimetic probes and molecular modeling suggest Alzheimer’s γ-secretases are intramembrane-cleaving aspartyl proteases. Biochemistry 38, 4720–4727.
Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., et al. (1999c) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517.
Wolozin B., Iwasaki K., Vito P., Ganjei J. K., Lacana E., et al. (1996) Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science 274(5293), 1710–1713.
Wong P. C., Zheng H., Chen H., Becher M. W., Sirinathsinghji D. J., et al. (1997) Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387(6630), 288–292.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolfe, M.S. γ-Secretase inhibitors as molecular probes of presenilin function. J Mol Neurosci 17, 199–204 (2001). https://doi.org/10.1385/JMN:17:2:199
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:17:2:199