Abstract
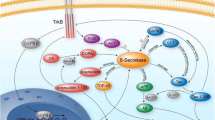
Major progress has recently been made in the characterization of the secretases involved in endoproteolytic processing of the Alzheimer’s disease (AD)-associated β-amyloid precursor protein, βAPP. βAPP is the precursor of the amyloid β-peptide, which is a major constituent of amyloid plaques in the brains of Alzheimer patients. It is now commonly believed that Aβ plays a pivotal role in the pathogenesis of AD, and that inhibiting the production of Aβ may help to treat or to prevent the disease. With β-secretase and the presenilins, two essential factors in the proteolytic generation of Aβ have now been identified. However, very little is still known about the biological function of the long-known βAPP. In this review we will discuss a novel putative function of βAPP in nuclear signaling, an activity, that βAPP may share with other presenilin substrates such as Notch.
Similar content being viewed by others
References
Borg J. P., Ooi J., Levy E., and Margolis B. (1996) The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell Biol. 16, 6229–6241.
Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J.R., et al. (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell 5, 207–216.
Buxbaum J. D., Liu K. N., Luo Y., Slack J. L., Stocking K. L., Peschon J. J., et al. (1998) Evidence that tumor necrosis factor a converting enzyme is involved in regulated a-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 273, 27,765–27,767.
Cao X. and Sudhof T. C. (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293, 115–120.
Capell A., Grunberg J., Pesold B., Diehlmann A., Citron M., Nixon R., et al. (1998) The proteolytic fragments of the Alzheimer’s disease-associated presenilin-1 form heterodimers and occur as a 100-150-kDa molecular mass complex. J. Biol. Chem. 273, 3205–3211.
Capell A., Steiner H., Romig H., Keck S., Baader M., Grim M. G., et al. (2000) Presenilin-1 differentially facilitates endoproteolysis of the β-amyloid precursor protein and Notch. Nat. Cell Biol. 2, 205–211.
De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., et al. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522.
Donoviel D. B., Hadjantonakis A. K., Ikeda M., Zheng H., Hyslop P. S., and Bernstein A. (1999) Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13, 2801–2810.
Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., and Russo T. (1995) The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J. Biol. Chem. 270, 30,853–30,856.
Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., and De Strooper B. (2000) Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell. Biol. 2, 461–462.
Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., et al. (1999) Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 96, 3922–3927.
Logeat F., Bessia C., Brou C., LeBail O., Jarriault S., Seidah N. G., and Israel A. (1998) The Notch 1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. USA 95, 8108–8112.
McLendon C., Xin T., Ziani-Cherif C., Murphy M. P., Findlay K. A., Lewis P. A., et al. (2000) Cell-free assays for γ-secretase activity. Faseb J. 14, 2383–2386.
McLoughlin D. M. and Miller C. C. (1996) The intracellular cytoplasmic domain of the Alzheimer’s disease amyloid precursor protein interacts with phosphotyrosine-binding domain proteins in the yeast two-hybrid system. FEBS Lett. 397, 197–200.
Mumm J. S. and Kopan R. (2000) Notch signaling: from the outside in. Dev. Biol. 228, 151–165.
Mumm J. S., Schroeter E. H., Saxena M. T., Griesemer A., Tian X., Pan D. J., et al. (2000) A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch 1. Mol. Cell 5, 197–206.
Pinnix I., Musunuru U., Tun H., Sridharan A., Golde T., Eckman C., et al. (2001) A novel γ-secretase assay based on detection of the putative C-terminal fragment-γ of amyloid β protein precursor. J. Biol. Chem. 276, 481–487.
Ray W. J., Yao M., Mumm J., Schroeter E. H., Saftig P., Wolfe M., et al. (1999) Cell surface presenilin-1 participates in the g-secretase-like proteolysis of Notch. J. Biol. Chem. 274, 36,801–36,807.
Sastre M., Steiner H., Fuchs K., Capell A., Condron M. M., Teplow D. B., and Haass C. (2001) Presenilin dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Reports (in press).
Schroeter E. H., Kisslinger J. A., and Kopan R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393, 382–386.
Selkoe D. J. (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399, A23-A31.
Steiner H., Duff K., Capell A., Romig H., Grim M. G., Lincoln S., et al. (1999) A loss of function mutation of presenilin-2 interferes with amyloid β-peptide production and Notch signaling. J. Biol. Chem. 274, 28,669–28,673.
Steiner H. and Haass C. (2000) Intramembrane proteolysis by presenilins. Nat. Rev. Mol. Cell Biol. 1, 217–224.
Steiner H., Kostka M., Romig H., Basset G., Pesold B., Hardy J., et al. (2000) Glycine 384 is required for presenilin-1 function and is conserved in polytopic bacterial aspartyl proteases. Nat. Cell Biol. 2, 848–851.
Struhl G. and Adachi A. (1998) Nuclear access and action of Notch in vivo. Cell 93, 649–660.
Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190.
Thinakaran G., Regard J. B., Bouton C. M., Harris C. L., Price D. L., Borchelt D. R., and Sisodia S. S. (1998) Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol. Dis. 4, 438–453.
Wolfe M. S., Xia W., Moore C. L., Leatherwood D. D., Ostaszewski B., Rahmati T., et al. (1999a) Peptidomimetic probes and molecular modeling suggest that Alzheimer’s γ-secretase is an intramembrane-cleaving aspartyl protease. Biochemistry 38, 4720–4727.
Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., and Selkoe D. J. (1999b) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517.
Yu G., Chen F., Levesque G., Nishimura M., Zhang D. M., Levesque L., et al. (1998) The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains β-catenin. J. Biol. Chem. 273, 16,470–16,475.
Yu G., Nishimura M., Arawaka S., et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407, 34–35.
Zhang L., Song L., Terracina G., Liu Y., Pramanik B., and Parker E. (2001) Biochemical characterization of the γ-secretase activity that produces β-amyloid peptides. Biochemistry 40, 5049–5055.
Zhang Z., Nadeau P. W. S., Donoviel D., Yuan M. A. B., and Yankner B. A. (2000) Presenilins are required for γ-secretase cleavage of βAPP and transmembrane cleavage of Notch-1. Nat. Cell. Biol. 2, 463–465.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Steiner, H., Haass, C. Nuclear signaling. J Mol Neurosci 17, 193–198 (2001). https://doi.org/10.1385/JMN:17:2:193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:17:2:193