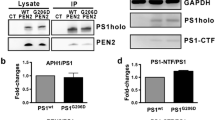
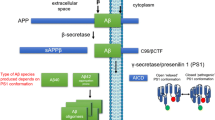
Abstract
Understanding mechanisms involved in the production of Aβ has long been the central focus of cell biologists engaged in molecular AD research. The discovery of two genes that encode homologous polytopic membrane proteins termed Presenilins (PS), has lead to several exciting recent findings on the proteolytic processes responsible for generating the COOH-terminus of Aβ. What we now know is that PS proteins play an important role in Aβ production and are considered one of the therapeutic targets. Here I have reviewed the vast literature on the biology of PS, especially focusing on PS endoproteolysis and the accumulation of stable PS derivatives that are likely the functional units.
Similar content being viewed by others
References
Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., et al. (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17, 1005–1013.
Brockhaus M., Grunberg J., Rohrig S., Loetscher H., Wittenburg N., Baumeister R., et al. (1998) Caspasemediated cleavage is not required for the activity of presenilins in amyloidogenesis and NOTCH signaling. Neuroreport 9, 1481–1486.
Capell A., Grunberg J., Pesold B., Diehlmann A., Citron M., Nixon R., et al. (1998) The proteolytic fragments of the Alzheimer’s disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J. Biol. Chem. 273, 3205–3211.
Capell A., Saffrich R., Olivo J. C., Meyn L., Walter J., Grunberg J., et al. (1997) Cellular expression and proteolytic processing of presenilin proteins is developmentally regulated during neuronal differentiation. J. Neurochem. 69, 2432–2440.
Capell A., Steiner H., Romig H., Keck S., Baader M., Grim M. G., et al. (2000) Presenilin-1 differentially facilitates endoproteolysis of the beta-amyloid precursor protein and Notch. Nat. Cell Biol. 2, 205–211.
Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., et al. (1997) Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72.
Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Pereztur J., et al. (1996) Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713.
Esler W. P., Kimberly W. T., Ostaszewski B. L., Diehl T. S., Moore C. L., Tsai J. Y., et al. (2000) Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat. Cell Biol. 2, 428–434.
Fraser P. E., Levesque G., Yu G., Mills L. R., Thirlwell J., Frantseva M., et al. (1998) Presenilin 1 is actively degraded by the 26S proteasome. Neurobiol Aging 19, S19-S21.
Goutte C., Hepler W., Mickey K. M., and Priess J. R. (2000) aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Development 127, 2481–2492.
Grunberg J., Walter J., Loetscher H., Deuschle U., Jacobsen H., and Haass C. (1998) Alzheimer’s disease associated presenilin-1 holoprotein and its 18–20 kDa C-terminal fragment are death substrates for proteases of the caspase family. Biochemistry 37, 2263–2270.
Guo Y., Livne-Bar I., Zhou L., and Boulianne G. L. (1999) Drosophila presenilin is required for neuronal differentiation and affects notch subcellular localization and signaling. J. Neurosci. 19, 8435–8442.
Hartmann H., Busciglio J., Baumann K. H., Staufenbiel M., and Yankner B. A. (1997) Developmental regulation of presenilin-1 processing in the brain suggests a role in neuronal differentiation. J. Biol. Chem. 272, 14,505–14,508.
Hendriks L., Thinakaran G., Harris C. L., De Jonghe C., Martin J. J., Sisodia S. S., and Van Broeckhoven C. (1997) Processing of presenilin 1 in brains of patients with Alzheimer’s disease and controls. Neuroreport 8, 1717–1721.
Honda T., Yasutake K., Nihonmatsu N., Mercken M., Takahashi H., Murayama O., et al. (1999) Dual roles of proteasome in the metabolism of presenilin 1. J. Neurochem. 72, 255–261.
Jacobsen H., Reinhardt D., Brockhaus M., Bur D., Kocyba C., Kurt H., et al. (1999) The influence of endoproteolytic processing of familial Alzheimer’s disease presenilin 2 on abeta42 amyloid peptide formation. J. Biol. Chem. 274, 35,233–35,239.
Kang D. E., Soriano S., Frosch M. P., Collins T., Naruse S., Sisodia S. S., et al. (1999) Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer’s disease-linked PS1 mutants in the beta-catenin-signaling pathway. J. Neurosci. 19, 4229–4237.
Kim T. W., Pettingell W. H., Hallmark O. G., Moir R. D., Wasco W., and Tanzi R. E. (1997a) Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J. Biol. Chem. 272, 11,006–11,010.
Kim T. W., Pettingell W. H., Jung Y. K., Kovacs D. M., and Tanzi R. E. (1997b) Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277, 373–376.
Kimberly W. T., Xia W., Rahmati T., Wolfe M. S., and Selkoe D. J. (2000) The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J. Biol. Chem. 275, 3173–3178.
Lau K. F., McLoughlin D. M., Standen C., and Miller C. C. (2000) X11 alpha and x11 beta interact with presenilin-1 via their PDZ domains. Mol. Cell Neurosci. 16, 557–565.
Leimer U., Lun K., Romig H., Walter J., Grunberg J., Brand M., and Haass C. (1999) Zebrafish (Danio rerio) presenilin promotes aberrant amyloid beta- peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry 38, 13,602–13,609.
Levitan D., Doyle T. G., Brousseau D., Lee M. K., Thinakaran G., Slunt H. H., et al. (1996) Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93, 14,940–14,944.
Lewis P. A., Perez-Tur J., Golde T. E., and Hardy J. (2000) The presenilin 1 C92S mutation increases abeta 42 production. Biochem. Biophys. Res. Comm. 277, 261–263.
Li X. and Greenwald I. (1996) Membrane topology of the C. elegans SEL-12 presenilin. Neuron 17, 1015–1021.
Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., DiMuzio-Mower J., et al. (2000) Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405, 689–694.
Loetscher H., Deuschle U., Brockhaus M., Reinhardt D., Nelboeck P., Mous J., et al. (1997) Presenilins are processed by caspase-type proteases. J. Biol. Chem. 272, 20,655–20,659.
Mercken M., Takahashi H., Honda T., Sato K., Murayama M., Nakazato Y., et al. (1996) Characterization of human presenilin 1 using N-terminal specific monoclonal antibodies: evidence that Alzheimer mutations affect proteolytic processing. FEBS Lett. 389, 297–303.
Morihara T., Katayama T., Sato N., Yoneda T., Manabe T., Hitomi J., et al. (2000) Absence of endoproteolysis but no effects on amyloid beta production by alternative splicing forms of presenilin-1, which lack exon 8 and replace D257A. Brain Res. Mol. Brain Res. 85, 85–90.
Murayama O., Honda T., Mercken M., Murayama M., Yasutake K., Nihonmatsu N., et al. (1997) Different effects of Alzheimer-associated mutations of presenilin 1 on its processing. Neurosci. Lett. 229, 61–64.
Nakano Y., Kondoh G., Kudo T., Imaizumi K., Kato M., Miyazaki J. I., et al. (1999) Accumulation of murine amyloidbeta42 in a gene-dosage-dependent manner in PS1 ‘knock-in’ mice. Eur. J. Neurosci. 11, 2577–2581.
Nishimura M., Yu G., Levesque G., Zhang D. M., Ruel L., Chen F., et al. (1999) Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nature Med. 5, 164–169.
Nowotny P., Gorski S. M., Han S. W., Philips K., Ray W. J., Nowotny V., et al. (2000) Posttranslational modification and plasma membrane localization of the Drosophila melanogaster presenilin. Mol. Cell Neurosci. 15, 88–98.
Okochi M., Eimer S., Bottcher A., Baumeister R., Romig H., Walter J., et al. (2000) A loss of function mutant of the presenilin homologue sel-12 undergoes abberant endoproteolysis in Caenorhabditis elegans and increased A-beta-42 generation in human cells. J. Biol. Chem. 275, 40,925–40,932.
Okochi M., Ishii K., Usami M., Sahara N., Kametani F., Tanaka K., et al. (1997) Proteolytic processing of presenilin-1 (PS-1) is not associated with Alzheimer’s disease with or without PS-1 mutations. FEBS Lett. 418, 162–166.
Oyama F., Sawamura N., Kobayashi K., Morishima-Kawashima M., Kuramochi T., Ito M., et al. (1998) Mutant presenilin 2 transgenic mouse: effect on an age-dependent increase of amyloid beta-protein 42 in the brain. J. Neurochem. 71, 313–322.
Passer B. J., Pellegrini L., Vito P., Ganjei J. K., and D’Adamio L. (1999) Interaction of Alzheimer’s presenilin-1 and presenilin-2 with Bcl-X(L). A potential role in modulating the threshold of cell death. J. Biol. Chem. 274, 24,007–24,013.
Podlisny M. B., Citron M., Amarante P., Sherrington R., Xia W., Zhang J., et al. (1997) Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol. Dis. 3, 325–337.
Ratovitski T., Slunt H. H., Thinakaran G., Price D. L., Sisodia S. S., and Borchelt D. R. (1997) Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J. Biol. Chem. 272, 24,536–24,541.
Sato N., Urano F., Leem J. Y., Kim S.-H., Li M., Donoviel D., Bernstein A., et al. (2000) Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nature Cell Biol. 2, 863–870.
Saura C. A., Tomita T., Davenport F., Harris C. L., Iwatsubo T., and Thinakaran G. (1999) Evidence that intramolecular associations between Presenilin domains are obligatory for endoproteolytic processing. J. Biol. Chem. 274, 13,818–13,823.
Saura C. A., Tomita T., Soriano S., Takahashi M., Leem J. Y., Honda T., et al. (2000) The non-conserved hydrophilic loop domain of presenilin (PS) is neither required for PS endoproteolysis nor enhanced Aβ42 production mediated by familial Alzheimer’s disease-linked PS variants. J. Biol. Chem. 275, 17,136–17,142.
Seeger M., Nordstedt C., Petanceska S., Kovacs D. M., Gouras G. K., Hahne S., et al. (1997) Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc. Natl. Acad. Sci. USA 94, 5090–5094.
Shirotani K., Takahashi K., Araki W., Maruyama K., and Tabira T. (2000) Mutational analysis of intrinsic regions of presenilin 2 that determine its endoproteolytic cleavage and pathological function. J. Biol. Chem. 275, 3681–3686.
Shirotani K., Takahashi K., Ozawa K., Kunishita T., and Tabira T. (1997) Determination of a cleavage site of presenilin 2 protein in stably transfected SH-SY5Y human neuroblastoma cell lines. Biochem. Biophys. Res. Comm. 240, 728–731.
Shirotani K., Takahashi K., and Tabira T. (1999) Effects of presenilin N-terminal fragments on production of amyloid beta peptide and accumulation of endogenous presenilins. Neurosci. Lett. 262, 37–40.
Song S., Ohba M., Saito Y., Honda T., Takashima A., and Takahashi H. (2000) Proteolytic processing and degradation of human presenilin-1 expressed in yeast. Neurosci. Lett. 282, 65–68.
Soriano S., Kang D. E., Fu M., Pestell R., Chevallier N., Zheng H., and Koo E. H. (2001) Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J. Cell Biol. 152, 785–794.
Steiner H., Capell A., Pesold B., Citron M., Kloetzel P. M., Selkoe D. J., et al. (1998) Expression of Alzheimer’s disease-associated presenilin-1 is controlled by proteolytic degradation and complex formation. J. Biol. Chem. 273, 32,322–32,331.
Steiner H., Kostka M., Romig H., Basset G., Pesold B., Hardy J., et al. (2000) Glycine 384 is required for presenilin-1 function and is conserved in bacterial polytopic aspartyl proteases. Nat. Cell Biol. 2, 848–851.
Steiner H., Romig H., Pesold B., Philipp U., Baader M., Citron M., et al. (1999) Amyloidogenic function of the Alzheimer’s disease-associated presenilin 1 in the absence of endoproteolysis. Biochemistry 38, 14,600–14,605.
Takahashi H., Mercken M., Honda T., Saito Y., Murayama M., Song S., and Takashima A. (1999) Impaired proteolytic processing of presenilin-1 in chromosome 14-linked familial Alzheimer’s disease patient lymphocytes. Neurosci. Lett. 260, 121–124.
Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190.
Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., et al. (1997) Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272, 28,415–28,422.
Thinakaran G., Regard J. B., Bouton C. M. L., Harris C. L., Price D. L., Borchelt D. R., and Sisodia S. S. (1998) Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol. Dis. 4, 438–453.
Tomidokoro Y., Ishiguro K., Igeta Y., Matsubara E., Kanai M., Shizuka M., et al. (1999) Carboxyl-terminal fragments of presenilin-1 are closely related to cytoskeletal abnormalities in Alzheimer’s brains. Biochem. Biophys. Res. Comm. 256, 512–518.
Tomita T., Takikawa R., Koyama A., Morohashi Y., Takasugi N., Saido T. C., et al. (1999) C terminus of presenilin is required for overproduction of amyloidogenic Ab42 through stabilization and endoproteolysis of presenilin. J. Neurosci. 19, 10,627–10,634.
Tomita T., Tokuhiro S., Hashimoto T., Aiba K., Saido T. C., Maruyama K., and Iwatsubo T. (1998) Molecular dissection of domains in mutant presenilin 2 that mediate overproduction of amyloidogenic forms of amyloid beta peptides. Inability of truncated forms of PS2 with familial Alzheimer’s disease mutation to increase secretion of Abeta42. J. Biol. Chem. 273, 21,153–21,160.
Tomita T., Watabiki T., Takikawa R., Morohashi Y., Takasugi N., Kopan R., et al. (2001) The first proline of PALP motif at the C terminus of presenilins is obligatory for stabilization, complex formation and {gamma}-secretase activities of presenilins. J. Biol. Chem. 276, 33,273–33,281.
van de Craen M., de Jonghe C., van den Brande I., Declercq W., van Gassen G., van Criekinge W., et al. (1999) Identification of caspases that cleave presenilin-1 and presenilin-2. Five presenilin-1 (PS1) mutations do not alter the sensitivity of PS1 to caspases. FEBS Lett. 445, 149–154.
Van Gassen G., De Jonghe C., Pype S., Van Criekinge W., Julliams A., Vanderhoeven I., et al. (1999) Alzheimer’s disease associated presenilin 1 interacts with HC5 and ZETA, subunits of the catalytic 20S proteasome. Neurobiol. Dis. 6, 376–391.
Weihl C. C., Ghadge G. D., Miller R. J., and Roos R. P. (1999) Processing of wild-type and mutant familial Alzheimer’s disease- associated presenilin-1 in cultured neurons. J. Neurochem. 73, 31–40.
Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., and Selkoe D. J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517.
Xu X., Shi Y., Wu X., Gambetti P., Sui D., and Cui M. Z. (1999) Identification of a novel PSD-95/Dlg/ZO-1 (PDZ)-like protein interacting with the C terminus of presenilin-1. J. Biol. Chem. 274, 32,543–32,546.
Yu G., Chen F., Levesque G., Nishimura M., Zhang D. M., Levesque L., et al. (1998) The Presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta-catenin. J. Biol. Chem. 273, 16,470–16,475.
Yu G., Chen F., Nishimura M., Steiner H., Tandon A., Kawarai T., et al. (2000) Mutation of conserved aspartates affects maturation of both aspartate mutant and endogenous presenilin 1 and presenilin 2 complexes. J. Biol. Chem. 275, 27,348–27,353.
Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and beta APP processing. Nature 407, 48–54.
Zhang D. M., Levitan D., Yu G., Nishimura M., Chen F., Tandon A., et al. (2000) Mutation of the conserved N-terminal cysteine (Cys92) of human presenilin 1 causes increased Abeta42 secretion in mammalian cells but impaired Notch/lin-12 signalling in C. elegans. Neuroreport 11, 3227–3230.
Zhang J., Kang D. E., Xia W., Okochi M., Mori H., Selkoe D. J., and Koo E. H. (1998) Subcellular distribution and turnover of presenilins in transfected cells. J. Biol. Chem. 273, 12,436–12,442.
Zhou J., Liyanage U., Medina M., Ho C., Simmons A. D., Lovett M., and Kosik K. S. (1997) Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport 8, 2085–2090.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thinakaran, G. Metabolism of Presenilins. J Mol Neurosci 17, 183–192 (2001). https://doi.org/10.1385/JMN:17:2:183
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:17:2:183