Abstract
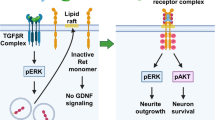
The nerve growth factor (NGF) trkA receptor is a transmembrane glycoprotein composed of a large extracellular ligand-binding region connected to the cytoplasmic tyrosine kinase region by a single transmembrane domain (TMD). To explore the role of TMD in the process of receptor activation, we substituted the hydrophobic amino-acid residue valine 432 with the charged amino-acid glutamic acid (designated V432E mutant) by utilizing in vitro site-directed mutagenesis. NIH 3T3 cells lacking endogenous NGF receptors were stably transfected with a pRc/CMV vector carrying either wild-type (trkA) or mutated (V432E) receptors. Stable transfectants were shown, using 125I-NGF binding and Western-blot analysis, to express the trkA recombinant receptors. Scatchard analysis revealed similar affinity for NGF in wild-type and V432E receptors. Although the level of basal trkA receptor tyrosine phosphorylation was higher in the mutant than in the wild-type, NGF stimulation of WT 11 and V432E transfectants resulted in a rapid increase in receptor tyrosine phosphorylation and of its intracellular adaptor protein SHC. In contrast to WT 11, V432E mutants showed very low levels of NGF-, and moderate levels of FGF-induced erks phosphorylation, respectively. Collectively, these findings suggest that a single substitution (V432E) in the trkA TMD results in a selective impairment of trkA-mediated erks signaling pathway.
Similar content being viewed by others
References
Baranski T. J., Herzmark P., Lichtarge O., Gerber B. O., Trueheart J., Meng E. C., et al. (1999) C5a receptor activation. Genetic identification of critical residues in four transmembrane helices. J. Biol. Chem. 274, 15,757–15,765.
Bonaventure J., Rousseau F., Legeai-Mallet L., LeMerrer M., Munnich A., and Maroteaux P. (1996) Common mutations in the fibroblast growth factor receptor 3 (FGFR3) gene account for achondroplasia, hypochondroplasia and thanatophoric dwarfism. Am. J. Med. Genet. 63, 148–154.
Briggs C. A., McKenna D. G., Monteggia L. M., Touma E., Roch J. M., Arneric S. P., et al. (1999) Gain of function mutation of the alpha 7 nicotinic receptor: distinct pharmacology of the human alpha 7V274T variant. Eur. J. Pharmacol. 366, 301–308.
Carpenter C. D., Ingrahm H. A., Cochet C., Walton G. M., Lazar C. S., Sowadski J. M., et al. (1991) Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J. Biol. Chem. 266, 5750–5755.
Clarke W. P. and Bond R. A. (1998) The elusive nature of intrinsic activity. Trends Pharmacol. Sci. 19, 270–276.
D’Hahan N., Jacquet H., Moreau C., Catty P., and Vivaudou M. (1999) A transmembrane domain of the sulfonylurea receptor mediates activation of ATP-sensitive K+ channels by K+ channel openers. Mol. Pharmacol. 56, 308–315.
Frattali A. L., Treadway J. L., and Pessin J. E. (1991) Evidence supporting a passive role of the insulin receptor transmembrane domain in insulin-dependent signal transduction. J. Biol. Chem. 266, 9829–9834.
Greene L. A. and Kaplan D. R. (1995) Early events in neurotrophin signaling via trk and p75 receptors. Curr. Opin. Neurobiol. 5, 579–587.
Guy P. M., Carraway K. L., and Cerione R. A. (1992) Biochemical comparison of the normal and oncogenic forms of insect cell-expressed neu tyrosine kinases. J. Biol. Chem. 267, 13,851–13,856.
Hoffman C., Moro S., Nicholas R. A., Harden T. K., and Jacobson K. A. (1999) The role of amino acids in extracellular loops of the human P241 receptor in surface expression and activation processes. J. Biol. Chem. 274, 14,639–14,647.
Jiang H., Shah S., and Hilt D. C. (1993) Characterization, sequence and expression of murine S100 beta gene. J. Biol. Chem. 268, 20,502–20,511.
Jiang H., Movsesyan V., Fink D. W. Jr., Fasler M., Whalin M., Katagiri Y., et al. (1997a) Expression of human p140trk receptors in p140trk-deficient, PC12/endothelial cells results in nerve growth factor-induced transduction and DNA synthesis. J. Cell Biochem. 66, 229–244.
Jiang H., Ulme St. D., Dickens G., Chabuk A., Lavarreda M., Lazarovici P., and Guroff G. (1997b) Both p140trk and p75NGFR nerve growth factor receptors mediate nerve growth factor-stimulated calcium uptake. J. Biol. Chem. 272, 6835–6837.
Kaplan D. R., Hempstead B. L., Martin Z. D., Chao M. V., and Parada L. F. (1991) The trk protooncogene product: a signal transducing receptor for nerve growth factor. Science 252, 554–558.
Kaplan D. R. and Stephens R. M. (1994) Neurotrophin signal transduction by the trk receptor. J. Neurobiol. 25, 1404–1417.
Karplus P. A. and Schulz G. E. (1985) Prediction of chain flexibility in proteins. Naturwissenschaften 72, 212,213.
Kashles O., Szapary D., Bellot F., Ullrich A., Schlessinger J., and Schmidt A. (1988) Ligand-induced stimulation of epidermal growth factor receptor mutants with altered transmembrane region. Proc. Natl. Acad. Sci. USA 85, 9567–9571.
Li Y., Mangasarian K., Mansukhani A., and Basilico C. (1997) Activation of FGF receptors by mutations in the transmembrane domain. Oncogene 14, 1397–1406.
Lin Z., Wang W., Kopajtic T., Revay R. S., and Uhl G. R. (1999) Dopamine transporter: transmembrane phenylalanine mutations can selectively influence dopamine uptake and cocaine analog recognition. Mol. Pharmacol. 56, 434–447.
Mardy S., Miura Y., Endo E., Matsuda I., Sztriha L., Frossard P., et al. (1999) Congenital insensitivity to pain with anhydrosis: novel mutations in the trkA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor. Am. J. Human Genet. 64, 1570–1579.
Martin-Zanca D., Barbacid M., and Parada L. F. (1990) Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 4, 683–694.
Martin-Zanca D., Hughes S. H., and Barbacid M. (1986) A human oncogene formed by the fusion of truncated tropamyosin and protein tyrosine kinase sequence. Nature 319, 743–748.
McNeil A., Larkins R., and Clark S. (1996) The uncoupling of SHC activation from distal intracellular signalling in a cell line which expresses a truncated human epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 218, 740–744.
Miloso M., Mazzotti M., Vass W. C., and Bequinot L. (1995) SHC and GRB2 are constitutively by an epidermal growth factor receptor with a point mutation in the transmembrane domain. J. Biol. Chem. 270, 19,557–19,562.
Scheer A., Fanelli F., Diviani D., Benedetti P. G., and Cotecchia S. (1999) Structure-function relationships of the alpha 1b-adrenergic receptor. Eur. Urol. 36(Suppl. 1), 11–16.
Takahashi K., Yonezawa K., and Nishimoto I. (1995) Insulin-like growth factor I receptor activated by a transmembrane mutation. J. Biol. Chem. 270, 19,041–19,045.
Thoenen H. and Barde Y. A. (1980) Physiology of nerve growth factor. Physiol. Rev. 60, 1284–1335.
Thompson S. A., Arden S. A., Marshall G., Wingrove P., Whiting P. J., and Wafford K. A. (1999) Residues in transmembrane domains I and II determine γ-aminobutyric acid type A receptor subtype-selective antagonism by furosemide. Mol. Pharmacol. 55, 993–999.
Wang S. Y. and Wang G. K. (1999) Batrachotoxin-resistant Na+ channels derived from point mutations in the transmembrane segment D4-S6. Biophys. J. 76, 3141–3149.
Watson F. L., Porcionatto M. A., Bhattacharyya A., Stiles C. D., and Segal R. A. (1999) TrkA glycosylation regulates receptor localization and activity. J. Neurobiol. 39, 323–326.
Wess J., Blin, N., Mutschler E., and Blumi K. (1995) Muscarinic acetylcholine receptors: structural basis of ligand binding and G protein coupling. Life Sci. 56, 915–922.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monshipouri, M., Jiang, H. & Lazarovici, P. NGF stimulation of erk phosphorylation is impaired by a point mutation in the transmembrane domain of trkA receptor. J Mol Neurosci 14, 69–76 (2000). https://doi.org/10.1385/JMN:14:1-2:069
Issue Date:
DOI: https://doi.org/10.1385/JMN:14:1-2:069