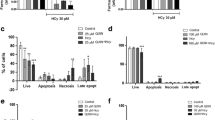
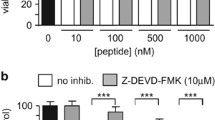
Abstract
Nitric oxide (NO) is an unstable radical produced during the oxidative deamination catalyzed by NO synthase (NOS) that converts l-arginine to l-citrulline. NO is also generated nonenzymatically from a group of compounds, called NO donors, such as sodium nitroprusside (SNP). NO directly or through its metabolites has been implicated in several disorders, including Alzheimer’s disease (AD). Since NO is a highly labile unstable free gas, we measured the stable end products, nitrite and nitrate (NOx). Here, we investigated the effect of SNP-mediated NO release in different cell types and its effect on the beta-amyloid precursor protein (βAPP). When different cell types were induced with SNP, a significant level of NOx was detected in a time and dose-dependent manner over the spontaneous release of NOx by SNP. The astrocytes, glial, and epithelial cell lines released significantly higher level of NOx as compared to neuronal cells following the exposure of SNP. The latter group of cells was more sensitive to NO-mediated cytotoxicity, as demonstrated by the lactate dehydrogenase assay. The SNP-mediated toxicity is known to be caused by the accumulation of cyanide ions and we report that the ability of cells to protect against it depends on the levels of nitric oxide metabolites. Cell lines, such as astrocytic and epithelial, that produce more NOx are better protected against the SNP-induced toxicity than the less NOx-protecting neuronal cell lines. The possibility of differential susceptibility of neurons and astrocytes resulting from the different content of reduced glutathione is also discussed. The release of NOx was prevented by cotreatment with a NO scavenger and superoxide dismutase but not by a NOS inhibitor. The activity of NOS was decreased when cytosolic extracts were incubated with SNP. In the conditioned medium of SNP-induced cells, the level of soluble βAPP (sAPP) was decreased, and this decrease was more apparent in neuronal than astrocytic cell lines. Taken together, these results suggest that the SNP-derived NO release is independent of the NOS pathway, that various cell types metabolize SNP differently, and that neuronal cell lines are more vulnerable with SNP treatment with lowered sAPP secretion. Since the neuronal cell lines lack a nitric-oxide-generated protective mechanism, we speculate that these cells may be the first targets of neurodegeneration by several toxic agents, including the cyanides and peroxynitrites.
Similar content being viewed by others
References
Anderson K. J., Dam K. D., Lee S., and Cotman C. W. (1988) Basic Fibroblast growth factor prevents death of lesioned cholinergic neuron in vivo. Nature 332, 360–361.
Beckman J. S., Beckman T. W., Chen J., Marshall P. M., and Freeman B. A. (1990) Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 87, 1620–1624.
Benzing W. C. and Mufson E. J. (1995) Increased number of NADPH-d-positive neurons within the substantia innominata in Alzheimer’s disease. Brain Res. 670, 351–355.
Bolanos J. P., Heales S. J. R., Land J. M., and Clark J. B. (1995) Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary cultures. J. Neurochem. 64, 1965–1972.
Bredt D. S. and Snyder S. H. (1992) Nitric oxide, a novel neuronal messenger. Neuron 8, 3–11.
Busse R. and Mulsch A. (1990) Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 275, 87–90.
Crow J. P. and Beckman J. S. (1996) The importance of superoxide in nitric oxide-dependent toxicity: evidence for peroxynitrite-mediated injury. Adv. Exp. Med. Biol. 387, 147–161.
Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., and Snyder S. H. (1993) Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci. 13, 2651–2661.
Devi L., Petanceska S., Liu R., Arbabha B., Bansinath M., and Garg U. (1994) Regulation of neuropeptide-processing enzymes by nitric oxide in cultured astrocytes. J. Neurochem. 62, 2387–2393.
Feinstein D. L., Galea E., Roberts S., Henrik B., Wang H., and Reis D. J. (1994) Induction of nitric oxide synthase in rat C6 glioma cells. J. Neurochem. 62, 315–321.
Galea E., Feinstein D. L., and Reis D. J. (1992) Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci. USA 89, 10,945–10,949.
Goldgaber D., Harris H. W., Hla T., Maciag T., Donnelly R. J., Jacobsen J. S., Vitek M. P., and Gajdusek C. D. (1989) Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA 86, 7606–7610.
Green L. C., Wagner D. A., Glowgowski J., Skipper P. L., Wisknok J. S., and Tannenbaum S. R. (1982) Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Anal. Biochem. 126, 131–138.
Griffin W. S. T., Stanley L. C., Ling C., White L., MacLeod V., Perrot L. J., White C. L., and Araoz C. (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 86, 7611–7615.
Hobbs A. J., Fukuto J. M., and Ignarro L. J. (1994) Formation of free nitric oxide from l-arginine by nitric oxide synthase: direct enhancement of generation by superoxide dismutase. Proc. Natl. Acad. Sci. USA 91, 10,992–10,996.
Hu J. and El-Fakahany E. E. (1993) Role of intercellular and intracellular communication by nitric oxide in coupling of muscarinic receptors to activation of guanylate cyclase in neuronal cells. J. Neurochem. 61, 578–585.
Kowaluk E. A., Seth P., and Fung H. (1992) Metabolic activation od sodium nitroprusside to nitric oxide in vascular smooth muscle. J. Pharmacol. Exp. Ther. 262, 916–922.
Kreye V. A. W. and Reske S. N. (1982) Possible site of the in vivo disposition of sodium nitroprusside in the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 320, 260–265.
Lahiri D. K. (1993) The stability of beta-amyloid precursor protein in nine different cell types. Biochem. Mol. Biol. Int. 29, 849–858.
Lahiri D. K., Lewis S., and Farlow M. R. (1994) Tacrine alters the processing of beta-amyloid precursor protein in different cell lines. J. Neurosci. Res. 37, 777–787.
Lahiri D. K. and Nall C. (1995) Promoter activity of the gene encoding the beta-amyloid precursor protein is up-regulated by growth factors, phorbol ester, retinoic acid and interleukin-1. Mol. Brain Res. 32, 233–240.
LeBlanc A. C., Xue R., and Gambetti P. (1996) Amyloid precursor protein metabolism in primary cell cultures of neurons, astrocytes, and microglia. J. Neurochem. 66, 2300–2310.
Leeuwenkamp O. R., Chin N. L. J., Van Der Mark E. J., Van Bennekom W. P., and Bult A. (1986) In vitro degradation of nitroprusside in relation to in vivo decomposition and mechanism of action. Int. J. Pharm. 33, 1–13.
Levi-Montalcini R. (1987) The nerve growth factor 35 years later. Science 237, 1154–1162.
Lindholm D., Heumann R., Meyer M., and Thoenen H. (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330, 658–659.
Maiese K., Boniece I., DeMeo D., and Wagner J. A. (1993) Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J. Neurosci. 13, 3034–3040.
Malenka R. C., Madison D. V., and Nicoll R. A. (1986) Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature 321, 175–177.
Mattson M. P., Tomaselli K. J., and Rydel R. E. (1993) Calcium-destabilizing and neurodegenerative effcets of aggregated β-amyloid peptide are attenuated by basic FGF. Brain Res. 621, 35–49.
Minc-Golomb D., Yadid G., Tsarfaty I., Resau J. H., and Schwartz J. P. (1996) In vivo expression of induclible nitric oxide synthase in cerebellar neurons. J. Neurochem. 66, 1504–1509.
Moncada S. and Higgs A. (1993) The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012.
Murphy S. and Guzybicki D. (1996) Glial NO. Normal and pathological roles. Neuroscientist 2, 90–99.
Nagase S., Takemura K., Ueda A., Hirayama A., Aoyagi K., Kondoh M., and Koyama A. (1997). A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D- or L-arginine. Biochem. Biophys. Res. Commun. 233, 150–153.
Peunova N. and Enlkolopov G. (1995) Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature 375, 68–73.
Rossi F. and Bianchini E. (1996) Synergistic induction of nitric oxide by β-amyloid and cytokines in astrocytes. Biochem. Biophys. Res. Commun. 225, 474–478.
Selkoe D. J. (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 8, 447–453.
Simmons M. L. and Murphy S. (1993) Cytokines regulate L-arginine dependent cyclic GMP production in rat glial cells. Eur. J. Neurosci. 5, 825–831.
Song W. and Lahiri D. K. (1997) Melatonin alters the metabolism of the beta-amyloid precursor protein in the neuroendocrine cell line PC12. J. Mol. Neurosci. 9, 75–92.
Slunt H. H., Thinakaran G., Koch C. V., Lo A. C. Y., Tanzi R. E., and Sisodia S. S. (1993) Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP). J. Biol. Chem. 269, 2637–2644.
Stopa E. G., Gonzalez A.-M., Chorsky R., Corona R. J., Alvarej J., Bird E. D., and Baird A. (1990) Basic fibroblast growth factor in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 171, 690–696.
Vitek M. P., Snell J., Dawson H., and Colton E. A., (1997) Modulation of nitric oxide production in human macrophages by apolipoprotein-E and amyloid-beta peptide. Biochim. Biophys. Res. Comm. 240, 391–394.
Wada K., Okada N., Yamamura T., and Koizumi S. (1996) Nerve growth factor induces resistance of PC12 cells to nitric oxide cytotoxicity. Neurochem. Int. 29, 461–467.
Yu O. and Chuang D. (1996) Inhibition of excitatory amino acid-induced phosphoinositide hydrolysis as a possible mechanism of nitroprusside neurotoxicity. J. Neurochem. 66, 346–354.
Zhang J. and Snyder S. H. (1995) Nitric oxide in the nervous system. Annu. Rev. Pharmacol. Toxicol. 35, 213–233.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghosh, C., Lahiri, D.K. Increased vulnerability of neuronal cell lines to sodium nitroprusside-mediated toxicity is caused by the decreased level of nitric oxide metabolites. J Mol Neurosci 13, 77–92 (1999). https://doi.org/10.1385/JMN:13:1-2:77
Issue Date:
DOI: https://doi.org/10.1385/JMN:13:1-2:77