Abstract
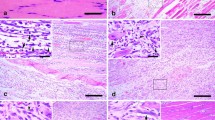
Tenascin-C is a multifunctional extracellular matrix glycoprotein with stimulatory and antiadhesive or inhibitory properties for axon growth. Its location and discontinous expression are restricted in innervated muscle tissues. Tenascin-C accumulated interstitially among human denervated muscle fibers and close to normal-sized fibers. To expand our knowledge of the expression of tenascin-C in human neuromuscular disorders, we investigated immunhistologically 20 human muscle specimens with type II myofiber atrophy of children and adults. Tenascin-C immunoreactivity in adult type II atrophy was frequent, and accumulation in children was sparse and weak. In both groups, tenascin-C immunoreactivity was found:
-
1.
Interstitially around normal-sized type II muscle fibers.
-
2.
Around atrophic type II muscle fibers.
-
3.
Around small-caliber myofibers with centrally located nuclei.
These results indicate that tenascin-Cimmunoreactivity: (1) is detectable around early denervated and reinnervated muscle fibers and, therefore, (2) may reflect in part the molecularly ongoing process of denervation and reinnervation in human type II fiber atrophy.
Similar content being viewed by others
References
Banker B. Q. and Engel A. G. (1994) Basic reactions of muscle, in Myology, 2nd ed., vol 1. (Engle A. G. and Franzini-Armstrong C., eds.) McGraw-Hill, New York, pp. 832–856.
Burch G. H., Gong Y., Liu W., Dettmann R. W., Curry C. J., Smith L., et al. (1997) Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nature Genet 17, 104–108.
Carnemolla B., Leprini A., Borsi L., Querze G., Urbini S., and Zardi L. (1996) Human tenascin-R: complete primary structure, pre-mRNA alternative splicing and gene localization on chromosome 1q23–q24. J. Biol. Chem. 271, 8157–8160.
Carpenter S. and Karpati G. (1984) Major general pathological reactions and their consequences on skeletal muscle cells, in Pathology of Skeletal Muscle (Carpenter S. and Karpati G., eds.) Churchill Livingstone, New York, pp. 101–112.
Chiquet-Ehrismann R. (1995) Inhibition of cell adhesion by anti-adhesive molecules. Curr. Opinion Cell Biol. 7, 715–719.
Connor E. A., Qin K., Yankelev H., and DeStefano D. (1994) Synaptic activity and connective tissue remodeling in denervated frog muscle. J. Cell Biol. 127, 1435–1445.
Daniloff J. K., Crossin K. L., Pincon-Raymond M., Murawsky M., Rieger F., and Edelman G. M. (1989) Expression of cytotactin in the normal and regenerating neuromuscular system. J. Cell Biol. 108, 625–635.
Dodd J. and Schuchardt A. (1995) Axon guidance: A compelling case for repelling growth cones. Cell 81, 471–474.
Erickson H. P. (1993) Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr. Opinion Cell Biol. 5, 869–875.
Faissner A. (1997) The tenascin gene family in axon growth and guidance. Cell Tissue Res. 290, 331–341.
Faissner A. and Kruse J. (1990) J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron 5, 627–637.
Faissner A., Götz B., Joester A., Wigger F., Scholze A., and Schütte K. (1996) Tenascin-C glycoproteins in neural pattern formation and regeneration. Semin. Neurosci. 8, 347–356.
Fischer D., Brown-Lüdi M., Schulthess T., and Chiquet-Ehismann R. (1997) Concerted action of tenascin-C domains in cell adhesion, anti-adhesion and promotion of neurite outgrowth. J. Cell Sci. 110, 1513–1522.
Gatchalian C. L., Schachner M., and Sanes J. R. (1989) Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin (J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J. Cell Biol. 108, 1873–1890.
Gulcher J. R., Alexakos M. J., Le Beau M. M., Lemons R. S., and Stefansson K. (1990) Chromosomal localization of the human hexabrachion (tenascin) gene and evidence for recent reduplication within the gene. Genomics 6, 616–622.
Gullberg D., Velling T., Sjörberg G., Salmivirta K., Gaggero B., Tiger C-F., et al. (1997) Tenascin-C expression correlates with macrophage invasion in Duchenne muscular dystrophy and myositis. Neuromuscl. Dis. 7, 39–54.
Hagios C., Koch M., Spring J., Chiquet M., and Chiquet-Ehrismann R. (1996) Tenascin-Y: a protein of novel domain structure is secreted by differentiated fibroblasts of muscle connective tissue. J. Cell Biol. 134, 1499–1512.
Irintechv A., Salvini T. F., Faissner A., and Wernig A. (1993) Differential expression of Tenascin after denervation, damage or paralysis of mouse soleus muscle. J. Neurocytol. 22, 955–965.
Itoh N. and Nagata S. (1993) A novel protein domain required for apoptosis. J. Biol. Chem. 268, 10,932–10,937.
Kirchner T., Tsartos S., Hoppe F., Schalke B., Wekerle H., and Müller-Hermelink H. K. (1988) Pathogenesis of myasthenia gravis. AJP 30, 268–280.
Langenfeld-Oster B., Faissner A., Irintchev A., and Wernig A. (1994) Polyclonal antibodies against NCAM and tenascin delay endplate reinnervation. J. Neurocytol. 23, 591–604.
Matsumoto K., Saga Y., Ikemura T., Sakakura T., and Chiquet-Ehrismann R. (1994) The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J. Cell Biol. 125, 483–493.
Mege R. M., Nicolet M., Pincoon-Raymond M., Murawsky M., and Rieger F. (1992) Cytotactin is involved in synaptogenesis during regeneration of the frog neuromuscular system. Dev. Biol. 149, 381–394.
Mendell J. R. and Engel W. K. (1971) The fine structure of type II muscle fiber atrophy. Neurology 21, 358–365.
Moses H. L., Yang E. Y., and Pietenpol J. A. (1990) TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63, 245–247.
Nies D. E., Hemesath T. J., Kim J-H., Gulcher J. R., and Stefansson K. (1991) The complete cDNA sequence of human hexabrachion (tenascin): a multidomain protein containing unique epidermal growth factor repeats. J. Biol. Chem. 266, 2818–2823.
Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., and Chiquet-Ehrismann R. (1988) Tenascin: cDNA cloning and induction by TGF-β. EMBO J. 7, 2977–2981.
Pedrosa-Domellöf F., Vitanen I., and Thornell L-E. (1995) Tenascin is present in human muscle spindles and neuromuscular junctions. Neurosci. Lett. 198, 173–176.
Sanes J. R., Schachner M., and Covault J. (1986) Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, Uvomorulin, Laminin, Fibronectin, and a Heparan Sulfate Proteoglycan) in embryonic, adult, and denervated skeletal muscle. J. Cell Biol. 102, 420–431.
Schoser B. G. H. and Goebel H. H. (1996) Tenascin in denervated human muscle. J. Neurol. Sci. 139, 203–209.
Schoser B. G. H., Löck G., and Blottner D. (1997) Partial loss of NADPH-diaphorase/nitric oxide synthase-complex in amyotrophic lateral sclerosis and human type II myofiber atrophy. Neurosci. Lett. 231, 163–166.
Settles D. L., Cihak R. A., and Erickson H. P. (1996) Tenascin-C expression in dystrophin-related muscular dystrophy. Muscle and Nerve 19, 147–154.
Telerman-Toppet N. and Coers C. (1975) Motor innervation in type II atrophy of skeletal muscle. J. Neurol. Sci. 25, 449–461.
Yamada H., Nakagawa M., Higuchi I., Ohkubo R., and Osame M. (1995) Type II muscle fibers are stained by anti-Fas antibody. J. Neurol. Sci. 134, 115–118.
Yonehara S., Ishii A., and Yonehara M. (1989) A cell-killing monoclonal antibody (Anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J. Exp. Med. 169, 1747–1756.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schoser, B.G.H., Faissner, A. & Goebel, H.H. Immunolocalization of tenascin-C in human type II fiber atrophy. J Mol Neurosci 13, 167–175 (1999). https://doi.org/10.1385/JMN:13:1-2:167
Issue Date:
DOI: https://doi.org/10.1385/JMN:13:1-2:167