Abstract
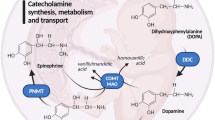
Expression of the gene encoding the epinephrine-synthesizing enzyme phenylethanolamine N-methyltransferase (PNMT) is regulated by hormonal and neural stimuli. Because the 5′-upstream regions of the PNMT do not contain sequences analogous to those demonstrated to convey neural regulation to the tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH) catecholamine-synthesizing enzyme genes, functional and biochemical analyses have been utilized to characterize PNMT promoter responses to cholinergic and depolarizing agents. In primary cultures of bovine adrenal medullary chromaffin cells, reporter gene expression from transiently transfected 3- and 0.9-kb-containing PNMT promoter constructs is stimulated approximately twofold by nicotine and muscarine. Depolarizing concentrations of K+ produce fourfold increases in expression. These responses are not detected with constructs containing the proximal 0.3-kb promoter, indicating that the regions between −273 and −877 bp convey neural responsiveness for the PNMT gene in bovine chromaffin cells. Electrophoretic mobility shift assays (EMSAs) with oligonucleotides encoding these regions of the PNMT promoter revealed distinctions in migration of nuclear protein complexes formed following treatment of chromaffin cells with nicotine, muscarine, or 50 mM K+. Thus, the PNMT promoter between 0.3 and 0.9 kb contains sequences capable of responding to cholinergic and depolarization stimuli. Moreover, these treatments influence the interactions of specific nuclear proteins with this region of the PNMT promoter.
Similar content being viewed by others
References
Baetge E. E., Behringer R. R., Messing A., Brinster R. L., and Palmiter R. D. (1988) Transgenic mice express the human phenylethanolamine N-methyltransferase gene in adrenal medulla and retina. Proc. Natl. Acad. Sci. USA 85, 3648–3652.
Batter D. K., D’ Mello S. R., Turzai L. M., Hughes H. B., Gioio A. E., and Kaplan B. B. (1988) The complete nucleotide sequence and structure of the gene encoding bovine phenylethanolamine N-methyltransferase. J. Neurosci. Res. 19, 367–376.
Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72, 248–254.
Carmichael S. and Stoddard S. L. (eds.) (1993) in The Adrenal Medulla 1989–1993. CRC, Boca Raton, pp. 185–196.
Carroll J. M., Evinger M. J., Goodman H. M., and Joh T. H. (1991) Differential and coordinate regulation of TH and PNMT mRNAs in chromaffin cell cultures by second messenger system activation and steroid treatment. J. Mol. Neurosci. 3, 75–83.
Craviso G. L., Hemelt V. B., and Waymire J. C. (1992) Nicotinic cholinergic regulation of tyrosine hydroxylase gene expression and catecholamine synthesis in isolated bovine adrenal chromaffin cells. J. Neurochem. 59, 2285–2296.
Dignam J. D., Lebovitz R. M., and Roeder R. G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489.
Ebert S. N. and Wong D. L. (1995) Differential activation of the rat phenylethanolamine N-methyltransferase gene by Sp-1 and Egr-1. J. Biol. Chem. 270, 17,299–17,305.
Ebert S. N., Ficklin M. B., Her S., Siddall B. J., Bell R. A. Ganguly K., et al. (1998) Glucocorticoid-dependent activation of neural crest factor AP-2: Stimulation of phenylethanolamine N-methyltransferase gene expression. J. Neurochem. 70, 2286–2295.
Edlund T., Walker M. D., Barr P. J., and Rutter W. J. (1985) Cell specific expression of the rat insulin gene: Evidence for the role of two distinct 5′ flanking elements. Science 230, 912–915.
Evinger M. J., Towle A. C., Park D. H., Lee P., and Joh T. H. (1992) Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro. Cell. Mol. Neurobiol. 12, 193–215.
Evinger M. J., Ernsberger P., Regunathan S., Joh T. H., and Reis D. J. (1994) A single transmitter regulates gene expression through two separate mechanisms: Cholinergic regulation of phenylethanolamine N-methyltransferase mRNA via nicotinic and muscarinic pathways. J. Neurosci. 14, 2106–2116.
Fader D. and Lewis E. J. (1990) Interaction of cyclic AMP and cell-cell contact in the control of tyrosine hydroxylase RNA. Mol. Brain. Res. 8, 25–29.
Fossom L. H., Carson C. D., and Tank A. W. (1991) Stimulation of tyrosine hydroxylase gene transcription rate by nicotine in rat adrenal medulla. Mol. Pharmacol. 40, 193–202.
Hiremagalur B., Nankova B., Nitahara J., Zeman R., and Sabban E. L. (1993) Nicotine increases expression of tyrosine hydroxylase gene—Involvement of protein kinase A-mediated pathway. J. Biol. Chem. 268, 23,704–23,711.
Hwang O., Park S. Y., and Kim K. S. (1997) Protein kinase A coordinately regulates both basal expression and cyclic AMP-mediated induction of three catecholamine-synthesizing enzyme genes. J. Neurochem. 68, 2241–2247.
Ishiguro H., Kim K. T., Joh T. H., and Kim K.-S. (1993) Neuron-specific expression of the human dopamine beta-hydroxylase gene requires both the cAMP response element and a silencer region. J. Biol. Chem. 268, 17,987–17,994.
Kilbourne E. J. and Sabban E. L. (1990) Differential effect of membrane depolarization on levels of tyrosine hydroxylase and dopamine beta-hydroxylase messenger RNAs in PC12 pheochromocytoma cells. Mol. Brain Res. 8, 121–127.
Kilbourne E. J., Nankova B. B., Lewis E. J., McMahon A., Osaka H., Sabban D. B., et al. (1992) Regulated expression of the tyrosine hydroxylase gene by membrane depolarization—identification of the responsve element and possible second messengers. J. Biol. Chem. 276, 7563–7569.
Kim K.-S., Lee M. K., Carroll J., and John T. H. (1993) Both the basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. J. Biol. Chem. 262, 4083–4089.
Kim K.-S., Ishiguro H., Tinti C., Wagner J., and Joh T. H. (1994) The cAMP-dependent protein kinase regulates transcription of the dopamine beta-hydroxylase gene. J. Neurosci. 14, 7200–7207.
Lazaroff M., Patankar S., Yoon S. O., and Chikaraishi D. M. (1995) The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic cell lines from transgenic mice. J. Biol. Chem. 270, 21,579–21,589.
Lee K. A. W., Bindereif A., and Green M. R. (1988) A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal. Technol. 5, 22–31.
McMahon A. and Sabban E. L. (1992) Regulation of expression of dopamine β-hydroxylase in PC12 cells by glucocorticoids and cyclic AMP analogues. J. Neurochem. 59, 2040–2047.
Morita K. and Wong D. L. (1996) Role of egr-1 in cholinergic stimulation of phenylethanolamine N-methyltransferase promoter. J. Neurochem. 67, 1344–1351.
Morita S., Kobayashi K., Hidaka H., and Nagatsu T. (1992) Organization and complete nucleotide sequence of the gene encoding mouse phenylethanolamine N-methlytransferase. Mol. Brain Res. 13, 313–319.
Morita K., Ebert S. N., and Wong D. L. (1995) Role of transcription factor egr-1 in phorbol ester-induced phenylethanolamine N-methyltransferase gene expression. J. Biol. Chem. 270, 11,161–11,167.
Nagamoto-Combs K., Piech K. M., Best J. A., Sun B., and Tank A. W. (1997) Tyrosine hydroxylase gene promoter activity is regulated by cyclic AMP-responsive element and AP1 sites following calcium influx. J. Biol. Chem. 272, 6051–6058.
Nordeen S. K. (1988) Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 6, 454–457.
Prestridge D. S. (1991) SIGNALSCAN. A computer program that scans DNA sequences for eukaryotic transcriptional elements. CABIOS 7, 203–206.
Ross M. E., Evinger M. J., Hyman S. E., Carroll J. M., Mucke L., Comb M., et al. (1990) Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase (PNMT) promoter using fusion genes introduced into chromaffin cells in primary culture. J. Neurosci. 10, 520–530.
Sambrook J., Fritsch E. F., and Maniatis T. (1989) Molecular Cloning—A Laboratory Manual, 2nd ed. Cold Springer Harbor Laboratory Press, Cold Spring Harbor, NY.
Sasaoka T., Kaneda N., Kurosawa Y., Fujita K., and Nagatsu T. (1989) Structure of human phenylethanolamine N-methyltransferase gene: existence of two types of mRNA with different transcription initiation sites. Neurochem. Int. 15, 555–565.
Seo H., Yang C., Kim H. S., and Kim K.-S. (1996) Multiple protein factors interact with the cis-regulatory elements of the proximal promoter in a cell-specific manner and regulate transcription of the dopamine β-hydroxylase gene. J. Neurosci. 16, 4102–4112.
Shaskus J., Greco D., Asnani L. P., and Lewis E. J. (1992) A bifunctional genetic regulatory element of the rat dopamine beta-hydroxylase gene influences cell type specificity and second messenger-mediated transcription. J. Biol. Chem. 267, 18,821–18,830.
Stachowiak M. K., Hong J. S., and Viveros O. H. (1990) Coordinate and differential regulation of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and proenkephalin mRNAs by neural and hormonal mechanisms in cultured bovine adrenal medullary cells. Brain Res. 510, 227–238.
Stachowiak M. K., Goc A., Hong J.-S., Poisner A., Jiang H.-K., and Stachowiak E. K. (1994) Regulation of tyrosine hydroxylase gene expression in depolarized non-transformed bovine adrenal medullary cells: second messenger systems and promoter mechanisms. Mol. Brain Res. 22, 309–319.
Swanson D. J., Zellmer E., and Lewis E. J. (1998) AP1 proteins mediate the cAMP response of the dopamine β-hydroxylase gene. J. Biol. Chem. 273, 24,065–24,074.
Tang K., Wu H., Mahata S. K., Taupenot L., Rozansky D. J., Parmer R. J., et al. (1996) Stimulus-transcription coupling in pheochromocytoma cells. J. Biol. Chem. 271, 28,382–28,290.
Tönshoff C., Hemmick L., and Evinger M. J. (1997) Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J. Mol. Neurosci. 9, 127–140.
Van Nguyen T., Kobierski L. A., Comb M. J., and Hyman S. E. (1990) The effect of depolarization on expression of the human enkephalin gene is synergistic with cAMP and dependent upon a cAMP-inducible enhancer. J. Neurosci. 10, 2825–2833.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, YS.E., Raia, G., Tönshoff, C. et al. Neural regulation of phenylethanolamine N-methyltransferase (PNMT) gene expression in bovine chromaffin cells differs from other catecholamine enzyme genes. J Mol Neurosci 12, 53–68 (1999). https://doi.org/10.1385/JMN:12:1:53
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:12:1:53