Abstract
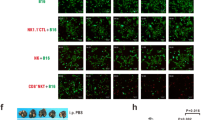
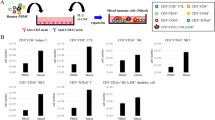
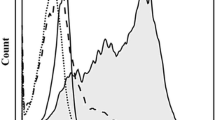
Co-culture of natural killer (NK) cells and dendritic cells (DCs) results in their reciprocal co-activation, and an enhancement of lysis of tumor target cells. The receptor:ligand pairings mediating this enhancement are unknown. Therefore, we investigated whether interactions of CD161, on NK cells, with Clrs, on DCs, might have a role in this effect. Blocking expression of CD 161B using siRNA resulted in a reduction in enhanced lytic activity following NK:DC co-culture. Conversely, blocking expression of CD161F with siRNA had no effect on enhanced lytic function following NK:DC co-culture. Blocking expression of ClrB/Ocil, a ligand for CD161B, resulted in a reduced level of enhanced lytic function following NK:DC co-culture. This is the first report of NK receptors responsible for interaction with DCs having a role in mediating enhanced lytic function following NK:DC interactions.
Similar content being viewed by others
References
Olcese L, Lang P, Vely F, et al: Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol 1996; 156:4531–4534.
Vely F, Vivier E: Conservation of structural features reveals the existence of a large family of inhibitory cell surface receptors and noninhibitory/activatory counterparts. J Immunol 1997; 159:2075–2077.
Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol 2003; 15:308–314.
Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH: Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2D subunits. J Immunol 1996; 157:4741–4745.
Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer (NK) cells and adherent lymphokine activated killer (ALAK) cells. J Exp Med 1989; 169:1373–1389.
Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1. 1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol 1991; 146:3662–3673.
Ryan JC, Niemi EC, Goldfien RD, Hiserodt JC, Seaman WE. NKR-P1, an activating molecule on rat natural killer cells, stimulates phosphoinositide turnover and a rise in intracellular calcium. J Immunol 1991; 147:3244–4350.
Campbell KS, Giorda R: The cytoplasmic domain of rat NKR-P1 receptor interacts with the N-terminal domain of p56(lck) via cysteine residues. Eur J Immunol 1997; 27:72–77.
Li J, Rabinovich BA, Hurren R, Shannon J, Miller RG: Expression cloning and function of the rat NK activating and inhibitory receptors NKR-P1A and-P1B. Int Immunol 2003; 15:411–416.
Yokoyama WM, Plougastel BF: Immune functions encoded by the natural killer gene complex. Nat Rev Immunol 2003; 3:304–316.
Ljunggren HG, Karre K: In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today 1990; 11:237–244.
Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol 2002; 55:221–228.
Zhou H, Kartsogiannis V, Hu YS, et al: A novel osteoblast-derived C-type lectin that inhibits osteoclast formation. J Biol Chem 2001; 276:14916–14923.
Zhou H, Kartsogiannis V, Quinn JM, et al: Osteoclast inhibitory lectin, a family of new osteoclast inhibitors. J Biol Chem 2002; 277:48808–48815.
Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol 2003; 4:801–807.
Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH: Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA 2004; 101:3527–3532.
Ryan JC, Niemi EC, Nakamura MC, Seaman WE: NKR-P1A is a target specific receptor that activates natural killer cell cytoticity. J Exp Med 1995; 181:1911–1915.
Lanier L, Chang C, Phillips JH: Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol 1994; 153:2417–2428.
Pinard D, Olsson NO, Chambers WH, Martin F: High expression of NKR-P1 is not an absolute requirement for natural killer activity in BDIX rats. Cancer Immunol Immunother 1996; 42:15–23.
Bezouska K, Yuen CT, O'Brien J, et al: Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature 1994; 372:150–157.
Kogelberg H, Montero E, Bay S, Lawson AM, Feizi T: Re-evaluation of monosaccharide binding property of recombinant soluble carbohydrate recognition domain of the natural killer cell receptor NKR-P1A. J Biol Chem 1999; 274:30335–30346.
Kogelberg H, Lawson AM, Muskett FW, Carruthers RA, Feizi T: Expression in Escherichia coli, folding in vitro, and characterization of the carbohydrate recognition domain of the natural killer cell receptor NKR-P1A. Protein Expr Purif 2000; 20:10–20.
Bezouska K, Vlahas G, Horvath O, et al: Rat natural killer cell antigen, NKR-P1, related to C-type animal lectins is a carbohydrate-binding protein. J Biol Chem 1994; 269:16945–16952.
Bezouska K, Sklenar J, Dvorakova J, et al: NKR-P1A protein, an activating receptor of rat natural killer cells, binds to the chitobioase core of uncompletely glycosylated N-linked glycans, and to linear chitooligmers. Biochem Biophys Res Comm 1997; 238:149–153.
Kogelberg H, Fenkiel TA, Homans SW, Lubineau A, Feizi T: Conformational studies on the selectin and natural killer cell receptor ligands sulfo- and sialo-lacto-N-fucopentoses (SuLNFPII and SLNFPII) using NMR spectroscopy and molecular dynamics simulations. Comparisons with the nonacidic parent molecule LNFPII. Biochem 1996; 35:1954–1964.
Krist P, Herkommerova-Rajnochova E, Rauvolfova J, et al: Toward an optimal oligosaccharide ligand for rat natural killer cell activation receptor NKR-P1. Biochem Biophys Res Comm 2001; 287:11–20.
Sememuk T, Krist P, Pavlicek J, et al: Synthesis of chitooligomer-based glycoconjugates and their binding to the rat natural killer cell activation receptor NKR-P1. Glycoconj J 2001; 18:817–826.
Poggi A, Costa P, Zocchi MR, Moretta L: NKRP1A molecule is involved in transendothelial migration of CD4+ human T cells. Immunol Lett 1997; 57:121–123.
Poggi A, Costa P, Zocchi MR, Moretta L: Phenotypic and functional analysis of CD4+ NKRP1A+ human T lymphocytes. Direct evidence that NKRP1A molecules is involved in transendothelial migration. Eur J Immunol 1997; 27:2345–2350.
Poggi A, Zocchi MR, Costa P, et al: IL 12-mediated NKRP1 up-regulation and consequent enhancement of endothelial transmigration of V delta 2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol 1999; 162:4349–4354.
Poggi A, Zocchi MR, Carosio R, et al: Transendothelial migratory pathways of V deltal+ TCR gamma delta+ and V delta2+ TCR gamma delta+ T cells from healthy donors and multiple sclerosis patients: Involvement of phosphatidylinositol 3 kinase at calcium calmodulin-dependent kinase II. J Immunol 2002; 168:6071–6077.
Fernandez NC, Lozier A, Flament C, et al: Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5:405–411.
Yu Y, Hagihara M, Ando K, et al: Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol 2001; 166(3):1590–1600.
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G: Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195:327–333.
Piccioli D, Sbrana S, Melandri E, Valiante NM: Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 2002; 195:335–341.
Miller G, Lahrs S, Dematteo RP: Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity. FASEB J 2003; 17:728–730.
Degli-Esposti MA, Smyth MJ: Close encounters of different kinds: dendritic cells and NK cells take center stage. Nat Rev Immunol 2005; 5:112–124.
Vujanovic NL, Herberman RB, Maghazachi AA, Hiserodt JC: Lymphokine-activated killer cells in rats. III. A simple method for the purification of large granular lymphocytes and their rapid expansion and conversion into lymphokine-activated killer cells. J Exp Med 1989; 167:15–29.
Brissette-Storkus CS, Kettel JC, Witham TF, Giezeman-Smits KM, Villa LA, Chambers WH: Flt-3 ligand drives differentiation of rat bone marrow derived dendritic cells (DCs) expressing OX62 and/or CD161. J Leuk Biol 2002; 71:941–949.
Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C: Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244). Blood 2005; 105:4722–4729.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, T., Flint, M.S., Webb, K.M. et al. CD161B: ClrB interactions mediate activation of enhanced lysis of tumor target cells following NK cell:DC co-culture. Immunol Res 36, 43–50 (2006). https://doi.org/10.1385/IR:36:1:43
Issue Date:
DOI: https://doi.org/10.1385/IR:36:1:43