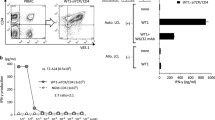
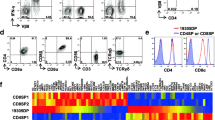
Abstract
Retroviral transfer of T cell antigen receptor (TCR) genes selected by circumventing tolerance to broad tumor- and leukemia-associated antigens in human leukocyte antigen (HLA)-A*0201 (A2.1) transgenic (Tg) mice allows the therapeutic reprogramming of human T lymphocytes. Using a human CD8×A2.1/Kb mouse-derived TCR specific for natural peptide-A2.1 (pA2.1) complexes comprising residues 81–88 of the human homolog of the murine double-minute 2 oncoprotein, MDM2(81–88), we found that the heterodimeric CD8αβ coreceptor, but not normally expressed homodimeric CD8αα, is required for tetramer binding and functional redirection of TCR-transduced human T cells. CD8+T cells that received a humanized derivative of the MDM2 TCR bound pA2.1 tetramers only in the presence of an anti-human-CD8 antibody and required more peptide than wild-type (WT) MDM2 TCR+ T cells to mount equivalent cytotoxicity. They were, however, sufficiently effective in recognizing malignant targets including fresh leukemia cells. Most efficient expression of transduced TCR in human T lymphocytes was governed by mouse as compared to human constant (C) αβ domains, as demonstrated with partially humanized and murinized TCR of primary mouse and human origin, respectively. We further observed a reciprocal relationship between the level of Tg WT mouse relative to natural human TCR expresion, resulting in T cells with decreased normal human cell surface TCR. In contrast, natural human TCR display remained unaffected after delivery of the humanized MDM2 TCR. These results provide important insights into the molecular basis of TCR gene therapy of malignant disease.
Similar content being viewed by others
References
Melief CJ, Toes RE, Medema JP, van der Burg SH, Ossendorp F, Offringa R: Strategies for immunotherapy of cancer. Adv Immunol 2000;75:235–282.
Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B: Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 1992;358:80–83.
Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ: Nucleo-cytoplasmatic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J 1998;17:554–564.
Freedman DA, Levine AJ: Regulation of the p53 protein by the MDM2 oncoprotein—thirty-eighth G.H.A. Clowes memorial award lecture. Cancer Res 1999;59:1–7.
Stanislawsk T, Voss RH, Lotz C, et al: Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol 2001;2:962–970.
Trotta R, Vignudelli T, Candini O, et al: BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell 2003;3:145–160.
Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA: Tolerance to p53 by A2.1-restricted T lymphocytes. J Exp Med 1997;185:833–841.
Vierboom MP, Nijman HW, Offringa R, et al: Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med 1997;186:695–704.
Kuball J, Schuler M, Antunes Ferreira E, et al: Generating p53-specific cytotoxic T lymphocytes by recombinant adenoviral vector-based vaccination in mice, but not man. Gene Ther 2002;9:833–843.
Lotz C, Ferreira EA, Drexler I, et al: Partial tyrosinase-specific self tolerance by HLA-A*0201-restricted cytotoxic T lymphocytes in mice and man. Int J Cancer 2004;108:571–579.
Zeh HJ, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC: High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol 1999;162:989–994.
Yang S Linette GP, Longerich S, Haluska FG: Antimelanoma activity of CTL generated from peripheral blood mononuclear cells after stimulation with autologous dendritic cells pulsed with melanoma gp 100 peptide G209-2M is correlated to TCR avidity. J Immunol 2002;169:531–539.
Gronsky MA, Boulter JM, Moskophidis D, et al: TCR affinity and negative regulation limit autommunity. Nat Med 2004;10:1234–1239.
Sherman LA, Hesse SV, Irwin MJ, La Face D, Peterson P: Selecting T cell receptors with high affinity for self-MHC by decreasing the contribution of CD8. Science 1992;258:815–818.
Pittet MJ, Rubio-Godoy V, Bioley G, et al: α3 domain mutants of peptide/MHC class I multimers allow the selective isolation of high avidity tumor-reactive CD8 T cells. J Immunol 2003;171:1844–1849.
Romero P, Dunbar PR, Valmori D, et al: Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytoloytic T lymphocytes. J Exp Med 1998;188:1641–1650.
Voss R-H, Kuball J, Theobald M: Designing TCR for cancer immunotherapy; in: Ludewig B, Hoffmann MW (eds): Adoptive Immunotherapy: Methods and Protocols, Totowa, NJ, Humana, 2004, pp. 229–256.
Kuball J, Schmitz FW, Voss RH, et al: Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity 2005;22:117–129.
Campanelli R, Palermo B, Garbelli S, et al: Human CD8 co-receptor is strictly involved in MHC-peptide tetramer-TCR binding and T cell activation. Int Immunol 2002;14:39–44.
Wooldridge L, Hutchinson SL, Choi EM, et al: Anti-CD8 antibodies can inhibit or enhance peptide-MHC class I (pMHCI) multimer binding: This is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface. J Immunol 2003;171:6650–6660.
Devine L, Hodson ME, Daniels MA, Jameson SC, Kavathas PB: Location of the epitope for an anti-CD8α antibody 53.6.7 which enhances CD8α-MHC class I interaction indicates antibody stabilization of a higher affinity CD8 conformation. Immunol Lett 2004;93:123–130.
Garcia KC, Degano M, Stanfield RL, et al: An αβ T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science 1996;274:209–219.
Holman PO, Walsh ER, Jameson SC: Characterizing the impact of CD8 antibodies on class I MHC multimer binding. J Immunol 2005;174:3986–3991.
Witte T, Spoerl T, Chang HC: The CD8β ectodomain contributes to the augmented coreceptor function of CD8αβ heterodimers relative to CD8αα homodimers. Cell Immunol 1999;191:90–96.
Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE: Rehermann B: Peripheral CD4+CD8+ T cells are differentiated effector memory cells with antiviral functions. Blood 2004;104:478–486.
Gangadharan D, Cheroutre H: The CD8 isoform CD8αα is not a functional homologue of the TCR co-receptor CD8αβ. Curr Opin Immunol 2004;16:264–270.
Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ: Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004;104:2840–2848.
Lanzavecchia A, Sallusto F: Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol 2002;2:982–987.
Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI: Efficient transfer of a tumor antigenreactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 1999;163:507–513.
Kessels HW, Wolkers MC, van den Boom M.D, van der Valk MA, Schumacher TN: Immunotherapy through TCR gene transfer. Nat Immunol 2001;2:957–961.
Heemskerk MH, Hoogeboom M, de Paus RA, et al: Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood 2003;102:3530–3540.
Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH: Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer, J Exp Med 2004;199:885–894.
Chamoto K, Tsuji T, Funamoto H, et al: Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res 2004;64:386–390.
Itano A, Salmon P, Kioussis D, Tolaini M, Corbella P, Robey E: The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J Exp Med 1996; 183:731–741.
Willemsen R, Ronteltap C, Heuveling M Debets R, Bolhuis R: Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR αβ gene transfer requires CD8α. Gene Ther 2005;12:140–146.
Morris EC, Tsallios A, Bendle GM, Xue SA, Stauss HJ: A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection. Proc Natl Acad Sci USA 2005;102:7934–7939.
Roszkowski JJ, Yu DC, Rubinstein MP, McKee MD, Cole DJ, Nishimura MI: CD8-independent tumor cell recognition is a property of the T cell receptor and not the T cell. J Immunol 2003;170:2582–2589.
Morgan RA, Dudley ME, Yu YY, et al: High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol 2003;171:3287–3295.
Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van, Besien K, Nishimura MI: Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res 2005;65:1570–1576.
Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA: Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol 2005;174:4415–4423.
Holler PD, Chlewicki LK, Kranz DM: TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol 2003;4:55–62.
Kessels HW, van den Boom MD, Sprits H, Hooijberg E, Schumacher TN: Changing T cell specificity by retroviral T cell receptor display. Proc Natl Acad Sci USA 2000;97:14578–14583.
Ho WY, Yee C, Greenberg PD: Adoptive therapy with CD8+ T cells: it may get by with a little help from its friends. J Clin Invest 2002;110:1415–1417.
Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP: CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003;421:852–856.
Sun JC, Williams MA, Bevan MJ: CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol 2004;5:927–933.
Wherry EJ, Teichgraber V, Becker TC, et al: Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003;4:225–234.
Bendle GM, Holler A, Pang LK, et al: Induction of unresponsiveness limits tumor protection by adoptively transferred MDM2-specific cytotoxic T lymphocytes. Cancer Res 2004;64:8052–8056.
Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW: Cutting edge: Tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol 2004;173:5923–5928.
Breedveld FC: Therapeutic monoclonal antibodies. Lancet 2000;355:735–740.
Berger C, Huang ML, Gough M, Greenberg PD, Riddell SR, Kiem HP: Nonmyeloablative immunosuppressive regimen prolongs in vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J Virol 2001;75:799–808.
Berger C, Blau CA, Huang ML, et al: Pharmacologically regulated Fas-mediated death of adoptively transferred T cells in a nonhuman primate model. Blood 2004;103:1261–1269.
Luznik L, Jalla S, Engstrom LW Iannone R, Fuchs EJ: Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 2001;98:3456–3464.
Ruggeri L, Capanni M, Urbani E, et al: Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097–2100.
Dudley ME, Wunderlich JR, Robbins PF, et al: Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002;298:850–854.
Call ME, Pyrdol J, Wiedmann M, Wucherpfenning KW: The organizing principle in the formation of the T cell receptor-CD3 complex. Cell 2002;111:67–979.
Author information
Authors and Affiliations
Corresponding author
Additional information
These two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Voss, RH., Kuball, J., Engel, R. et al. Redirection of T cells by delivering a transgenic mouse-derived MDM2 tumor antigen-specific TCR and its humanized derivative is governed by the CD8 coreceptor and affects natural human TCR expression. Immunol Res 34, 67–87 (2006). https://doi.org/10.1385/IR:34:1:67
Issue Date:
DOI: https://doi.org/10.1385/IR:34:1:67