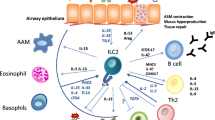
Abstract
A large body of research supports a pathogenic role of Th2 cells in allergic diseases such as asthma. These disorders are characterized by recruitment to selected peripheral tissues of a mixed leukocyte inflammatory infiltrate including a predominant e osinophil component. The development of this inflammatory response is dependent on accumulation of Th2 cellsin the affected tissues. Our studies aim to define the mechanisms that control the development of this tissue inflammatory response, focusing particularly on the mechanisms that recruit Th2 cells to the lung and airway. We have found that Th2 cells are their own poorly competent for antigen-induced recruitment to the lung. By contrast, Th1 cells are avidly recruited to the lungs in response to airway antigen challenge. More important, recruitment of Th1 cells to the lung resulted in enhanced recruitment of Th2 cells to this tissue. The increased. Th1 cell-induced recruitment of Th2 cells was associated with upregulation of endothelial vascular cell adhesion molecule-1 (VCAM-1) expression in airway-associated endothelial cells and could be largely blocked by systemic treatment with a monoclonal anti-VCAM-1 antibody. Systemic blocking of tumor necrosis factor (TNF) also blunted the airway inflammatory response. The prominent roles of TNF and VCAM-1 in recruitment of Th2 cells suggested that an inflammatory microenvironment was essential for the recruitment of Th2 cells. Infact, recruitment of Th2 cells to the airway could be induced in an antigen-independent fashion by proinflammatory stimuli such as intranasal in stillation of endotoxin. This antigen nonspecificity of the Th2 cell recruitment suggested a model in which Th2 cell recruitment is in response to general inflammatory signals rather than to antigen itself. This model provides an explanation for the clinical observation that bacterial or viral respiratory tract infections are associated with disease exacerbations in allergic asthmatics. More generally, these data imply that Th2 cells, like other leukocytes, are recruited efficiently to site of tissue inflammation, and that these nonspecifically recruited Th2 cells have substantial potential to modulate local inflammatory processes.
Similar content being viewed by others
References
Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI: NIH conference. Airway inflammation. Ann Intern Med 1995;123:288–304.
Bentley AM, Meng Q, Robinson DS, Hamid Q, Kay AB, Durham SR: Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/ macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopics asthmatics. Am J RespirCell Mol Biol 1993;8:35–42.
Kay AB: T-cells asorchestrators of the asthmatic response. Ciba Found Symp 1997;206:56–67.
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB: Predominant TH2-like bronchoalveolar T-lymphocyte population inatopic asthma. N Engl J Med 1992;326:298–304.
Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH: T cell cytokine profile evaluated at the single cell level in BAL. and blood in allergic asthma. Am J Respir Cell Mol Biol 1996;14: 319–326.
Ying S, Durham SR, Corrigan CJ, Hamid Q, Kay AB: Phenotype of cells expressing mRNA for TH2-type (interleuk in 4 and interleukin 5) and TH1-type (interleukin 2 and interferon gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol 1995;12:477–487.
Corne JM, Holgate ST: Mechanisms of virus induced exacerbations of asthma. Thorax 1997;52: 380–389.
Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM: Interleukin 4, but not interleukin-5 or eosinophils, is requied in a murine model of acute airway hyperreactivity. J Exp Med 1996;183:109–117.
Lee JJ, McGrarry MP, Farmer SC, et al: Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med 1997;185:2143–2156.
Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K: Induction of airway mucus production by Thelper 2 (Th2) cells: acriticalrole for interleukin 4 in cell recruitment but not mucus production. J Exp Med 1997;186:1737–1747.
Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K: Th2-induced airway mucus production isdependenton IL-4R α, but not on eosinophils. J Immunol 1999;162:6178–6183.
Cohn L, Herrick C, Niu N, Homer R, Bottomly K: IL-4 promotes airway eosinophilia by suppressing IFN-γ production: defining a novel role for IFN-γ in the regulation of allergic airway inflammation. J Immunol 2001;166: 2760–2767.
Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M: Interleukin 12 inhibits antigen-induced airway hyperre-sponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med 1995;182:1527–1536.
Bruselle GG, Kips JC, Peleman RA, Joos GF, Devos RR, Tavernier JH, Pauwels RA: Role of IFN-gamma in the inhibition of the allergic airway inflammation caused by IL-12. Am J Respir Cell Mol Biol 1997;17:767–771.
Koh YI, Choi IS, Kim WY: BCG infection in allergen-presensitized rats suppresses Th2 immune response and prevents the development of allergic asthmatic reaction. J Clin Immunol 2001;21:51–59.
Wills-Karp M: Immunologic basis of antigen-induced airway hyper-responsiveness. Annu Rev Immunol 1999;17:255–281.
Randolph DA, Carruthers CJL, Szabo SJ, Murphy KM, Chaplin DD: Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol 1999;162:2375–2383.
Murphy KM, Heimberger AB, Loh DY: Induction by antigen of intrathymic apoptosis of CD4+ CD8+TCRlo thymocyles in vivo. Science 1990;250:1720–1723.
Fu YX, Huang G, Matsumoto M, Molina H, Chaplin DD: Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc Natl Acad Sci USA 1997;94:5739–5743.
Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD: Lymphotox in-α, supports development of splenic folicularstructure that is required for IgG responses. J Exp Med 1997;185:2111–2120.
Fu YX, Chaplin DD: Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999;17:399–433.
Fu YX, Huang C, Wang Y, Chaplin DD: Lymphotox in-α-dependent spleen microenvironment is required for the generation of memory B cells and for their subsequentantigen-induced activation. J Immunol 2000;164:2508–2514.
Matsumoto M, Fu YX, Molina H, Huang G, Kim J, Thomas D, Nahm MH, Chaplin DD: Distinct roles of lymphotoxin-α, and the type I tumornecrosis factor (TNF) receptor in the establishment of follicular dendritic cells from non-bone marrow-derived cells. J Exp Med 1997;186:1997–2004.
Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM: Differential regulation of T helper phenotype development by interleukins 4 and 10 in an α,β T-cellreceptor transgenic system. Proc Natl Acad Sci USA 1992;89: 6065–6069.
Hansen G, Berry G, DeKruyff RH, Umetsu DT: Allergen-specific Th1 cells fail tocounterbalance Th2 cell-induced airway hy perreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–183.
Kline JN: Effects of CpG DNA on the Th1/Th2 balance in asthma. Curr Top Microbiol Immunol 2000;247:211–225.
Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K: Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol 2000;164: 5575–5582.
Fukuda T, Fukushima Y, Numao T, Ando N, Arima M, Nakajima H, Sagara H, Adachi T, Motojima S, Makino S: Role of interleukin-4 and vascular cell adhesion molecule-1 in selective eosinophil migration into the airways inallergic asthma. Am J Respir Cell Mol Biol 1996;14:84–94.
Yang LY, Cohn L, Zhang DH, Homer R, Ray A, Ray P: Essential role of nuclear factor kappa-B in the induction of eosinophilia in allergic airway inflammation. J Exp Med 1998;188:1739–1750.
Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A: P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 1997;385:81–83.
Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hammann A, Vestweber D: P-selectin glycoprotein ligand-1 (PSGL-1) on Thelper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med 1997;185:573–578.
Randolph DA, Stephens R, Carruthers CJL, Chaplin DD: Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest 1999;104:1021–1029.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaplin, D.D. Cell cooperation in development of eosinophil-predominant inflammation in airways. Immunol Res 26, 55–62 (2002). https://doi.org/10.1385/IR:26:1-3:055
Issue Date:
DOI: https://doi.org/10.1385/IR:26:1-3:055