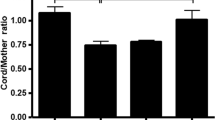
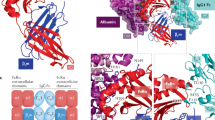
Abstract
This review describes the evolution of our knowledge of the transmission of immunoglobulin G (IgG) from mother to infant and the factors which regulate the persistence of IgG in the circulation. These apparently unrelated processes involve the same Fc receptor, FcRn (n=neonatal). FcRn appears to carry out these diverse roles by binding to IgG and then either transporting the bound IgG across cells (transcytosis) or recycling its cargo back to the cell surface (control of catabolism). IgG that is taken up by cells in the absence of binding to FcRn undergoes degradation. Thus, FcRn is the “protective” receptor that servesto maintain IgG homeostasis and deliver IgGs across cellular barriers.
Similar content being viewed by others
Literatur
Endo T, Wright A, Morrison SL, Kobata A: Glycosylation, of the variable region of immunoglobulin G—site specific maturation of the sugar chains. Mol Immunol 1995;32:931–940.
Ward ES, Ghetie V: The effector functions of immunoglobulins: implications for therapy. Ther Immunol 1995;2:77–94.
Blumberg RS, Koss T, Story CM, Barisani D, Polischuk J, Lipin A, et al: A major histocompatibility complex class 1-related Fc receptor for IgG on rat hepatocytes. J Clin Invest 1995;95:2397–2402.
Telleman P, Junghans RP: The role of the Brambell receptor (FcRB) in liver: protection of endocytosed immunoglobulin G (IgG) from catabolism in hepatocytes rather than transport of IgG to bile. Imunology 2000;100:245–251.
Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, et al: Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology 1997;92:69–74.
Cianga P, Medesan C, Richandson JA, Ghetie V, Ward ES: Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol 1999; 29:2515–2523.
Brambell FWR: The Transmission of Passive Immunity from Mother to Young. Amsterdam, North Holland Publ Corp, 1970.
Jones, EA, Waldmann TA: The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest 1972; 51:2916–2927.
Waldmann TA, Strober W: Metabolism of Immunoglobulins. Prog Allergy 1969;13:1–110.
Ghetie V, Hublanrd JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally shortserum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 1996;26: 690–696.
Junghans RP, Anderson CL: The protection receptor for IgG catabolism is the beta 2-micro globulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA 1996;93:5512–5516.
Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE: Increased, clearanceof IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology 1996;89:573–578.
Rodewald R: Intestinal transport of antibodies in the newborn rat. J Cell Biol 1973;58:189–211.
Rodewald R: pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol 1976;71:666–669.
Rodewald R, Kraehenbuhl JP: Receptor-mediated transport of IgG. J Cell Biol 1984;99: 159s-164s.
Simister NE, Rees AR: Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol 1985;15: 733–738.
Roberts DM, Guenthert M, Rodewald R: Isolation and characterization of the Fc receptor from the fetalyolksac of the rat. J Cell Biol 1990;111:1867–1876.
Rodewald R, Abrahamson DR: Receptor-mediated transport of IgG across the intestinal epithelium of the neonatal rat. Ciba Found Symp 1982;209–232.
Simister NE, Mostov KE: An Fc receptor structurally related to MHC class I antigens. Nature 1989;337:184–187.
Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE: Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol 1995;154: 6246–6251.
Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ: Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995;34:14,649–14,657
Popov S, Hubbard JG, Kim J, Ober, B, Ghetie, V, Ward ES: The stoichiometry andaffinity of the interaction of murine Fc fragments with the MHC class I-related receptor, FcRn. Mol Immunol 1996;33: 521–530.
Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R: IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur J Immunol 1999;29:733–744.
Praetor A, Ellinger I, Hurziker W: Intracellular traffic of the MHC class 1-like IgG Fc receptor, FcRn, expressed in epithelial MDCK cells. J Cell Sci 1999;112:2291–2299.
McCarthy KM, Yoong Y, Simister NE: Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci 2000;113:1277–1285.
Antohe F, Radulescu L, Gafencu A, Ghetie V, Simionescu M: Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol 2001;62:93–105.
Stefaner I, Praetor A, Hunziker W: Nonvectorial surface transport, endocytosis via a di-leucine-based motif, and bidirectional transcytosis of chimera encoding the cytosolictail of rat FcRn expressed in Madin-Darby canine kidney cells. J Biol Chem 1999;274:8998–9005.
Wu Z, Simister NE: Tryptophan-and dilecine-based endocytosis signals in the neonatal Fe receptor. J Biol Chem 2001;114:1591–1598.
Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transportinapolarized human intestinal epithelial cell line. J Clin Invest 1999;104:903–911.
Schultze HE, Heremans JF: Molecular Biology of Human Proteins. Amsterdam: Elsevier, 1966; pp 450–455.
Ghetie V and Buzila L: Relationship between the pH-dependent conformation change of rabbit immunoglobulin G and its digestibility by papain. Rev Rom Biochim 1972;9:125–133.
Waldman TA and Ghetie V: Catabolism of Immunoglobulins. Prog Immunol 1971:1:1187–1191.
Ashwell G, Morell AG: The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzynol 1974;41:99–128.
Robinson AB, McKerrow JH, Cary P: Controlled deamidation of peptides and proteins: an experimental hazard and a possible biological timer. Proc Natl Acad Sci USA 1970;66:753–757.
Wallevik K: Spontaneous in vivo isomerization of bovine serum albumin as a determinant of its normal catabolism. J Clin Invest 1976;57:398–407.
Margineanu I, Ghetie V: A selective model of plasma protein catabolism. J Theor Biol 1981;90:101–110.
Margineanu I, Ghetie V: A probabilistic approach to ageing and elimination of senescent plasma protein. Mech Ageing Dev 1983; 23:297–305.
Dobre MA, Marx A, Ghetie V: Isolation of a rabbit IgG fraction with cytophilic properties. J Immunol Methods 1983;59:339–348.
Sulica A, Gherman M, Medesan C, Sjoquist J, Ghetie V: IgG-binding sites on macrophage cell membrane. I. Identification of two distinct Fc receptors on mouse peritoneal macrophages. Eur J Immunol 1979;9:979–984.
Sulica A, Herberman RB: Cytophilic immunoglobulins revisited via natural killer cells. FASEBJ 1996;10:1495–1504.
Ghetie V, Onica D, Lenkei R, Margineanu I: An immunological hypothesis for plasma protein catabolism. Mech Ageing Dev 1981;17:27–39.
Gregoriadis G: The role of sialic acid in the catabolism of plasma glycoproteins. In: Structure and function of antibodies (ed. Allison AC) New York: Plenum Press, 1976, pp. 145–163.
Turner MW: Structure and Fucction of Antibodies. New York: Wiley, 1981, p. 39.
Wawrzynczak EJ, Cumber AJ, Parnell GD, Jones PT, Winter G: Blood clearance in the rat of a recombinant mouse monoclonal antibody lacking the N-linked oligosaccharidesside chain of the CH2 domains. Mol Immunol 1992;29:213–220.
Vlassara H, Brownlee M, Cerami A: High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci USA 1985;82:5588–5592.
Kennedy DM, Skillen AW, Self CH: Glycation increases the vascularcleara rate of IgG in mice. Clin Exp Immunol 1993;94:447–451.
Brambell EWR, Hemmings WA, Morris IG: A Theoretical Model of γ-globulin catabolism. Nature 1964;203:1352–1355.
Ghetie V: Cellular events which are the basis of catabolism of immunoglobulins. Microbiol Parazitol Epidemiol (Bucharest) 1972;17:317–324.
Wallace KH, Rees AR: Studies on the immunoglobulin-G Fc-fragment receptor from neonatal rat small intestine. Biochem J 1980;188;9–16.
Mariani G, Strober W: Fc Receptors and the Action of Antibodies. Washington, DC: Am Soc Microbiol 1990;94–180.
Borvak J, Richardson J, Medesan C, et al: Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 1998;10:1289–1298.
Junghans RP: Finally! The Brambell receptor (FcRB): Mediator of transmission of immunity and protection from catabolism for IgG. Immunol Res 1997;16:29–57.
McFarlane AS: Catabolism of plasma proteins. Lancet 1963;1:131–132.
Ghitescu L, Fixman A, Simionescu M, Siminnescu N: Specific binding sites for albumin restricted to plasmalemmal vesicles of continous capillary endothelium: receptor-mediated transcytosis. J Cell Biol 1986;102:1304–1311.
Schnitzer JE, Oh P: Albondin-mediated capillary permeability to albumin. Differential roleo freceptors in endothelia transcytosis and endocytosis of native and modified albumins. J Biol Chem 1994;269:6072–6082.
Freeman T, Gordon AH, Humphrey JH: Distinction between catabolism of native and denatured proteins of the isolated perfused liver after carbon loading. Br J Exp Pathol 1958;39:459–471.
Dima S, Medesan C, Mota G, Moraru I, Sjoquist J, Ghetie V: Effect protein A and its fragment B on the catabolic and Fc receptor sites of IgG. Eur J Immunol 1983; 13:605–614.
Deisenhofer J: Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcusaureaus at 2.9-and 2.8-A resolution. Biochemistry 1981;20:2361–2370.
Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C: Sequences of proteins of immunological interst. U.S. Dept. of Health and Human Services, 1991.
Kim JK, Tsen MF, Ghetie V, Ward ES: Localization of the site of the murine IgGI molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol 1994;24:2429–2434.
Kim JK, Tsen MF, Ghetie V, Ward ES: Identifying amino acid residues that influence plasma clerrance of murine IgG1 fragments by site-directed mutagenesis. Eur J Immunol 1994;24:542–548.
Medesan C, Radu C, Kim JK, Ghetie V, Ward ES: Localization of the site of the IgG molecule that regulates maternofetal transmission in mice. Eur J Immunol 1996; 26:2533–2536.
Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES: Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol 1997;158:2211–2217.
Burmeister WP, Huber AH, Bjorkman PJ: Crystal structure of the complex of neonatal Fc receptor with Fc. Nature 1994;372:379–383.
Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES: Mapping the site on human IgG for binding of the MHC class 1-related receptor, FcRn. Eur J Immunol 1999;29:2819–2825.
Christianson GJ, Brooks W, Vekasi S, et al.: Beta 2-microglobulin-deficient mice are protected from hypergammaglobulinemia and have defective antibody responses because of increased IgG catabolism. J Immunol 1997;159:4781–4792.
Kim JK, Tsen MF, Ghetie V, Ward ES: Catabolism of the murine IgG1 molecule: evidence that both CH2–CH3 domain interfaces are required for persistence of IgG1 in the circulation of mice. Scand J Immunol 1994;40:457–465.
Sanchez LM, Penny DM, Bjorkman PJ: Stoichiometry of the interaction between the major histocompatibility complex-related Fc receptor and its Fc ligand. Biochemistry 1999;38:9471–9476.
Martin WL, Bjorkamm PJ: Characterization of the 2:1 complex between the class I MHC-related Fc receptor and its Fc ligand in solution. Biochemistry 1999;38:12,639–12,647.
Schuck P, Radu CG and Ward ES: Sedimentation equilibrium analysis of recombinant mouse FcRn with murine IgG1. Mol Immunol 1999;36:1117–1125.
Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES: Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol 1997;15:637–640.
Nezlin R: Internal movements in immunoglobulin molecules. Adv Immunol 1990;48:1–40:1–40.
Zheng Y, Shopes B, Holowka D, Baird B: Dynamic conformations compared for IgE and IgG1 insolution and bound to receptors. Biochemistry 1992;31:7446–7456.
Medesan C, Cianga P, Mummert M, Stanescu D, Ghetie V, Ward ES: Comparative studies of rat IgG to further delineate the Fc: FcRn interaction site. Eur J Immunol 1998; 28:2092–2100.
Kim JK, Tsen MF, Ghetie V, Ward ES: Evidence that the hinge region plays a role in maintaining serum levels of the murine IgGI molecule. Mol Immunol 1995;32:467–475.
Zuckier LS, Chang CJ, Scharff MD, Morrison SL: Chimeric human-mouse 1gG antibodies with shuffled constant region exons demonstrate that multiple domains contribute to in vivo half-life. Cancer Res 1998;58:3905–3908.
Morell A, Terry WD, Waldmann TA: Metabolic properties of IgG subclasses in man. J Clin Invest 1970;49:673–680.
Zuckier LS, Georgescu L, Chang CJ, Scharff MD, Morrison SL: The use of severe combined immunodeficiency mice to study the metabolism of human immunoglobulin G. Cancer 1994;73:794–799.
Bazin R, Boucher G, Monier G, et al.: Use of hu-IgG-SCID mice to evaluate the in vivo stability of human monoclonal IgG antibodies. J Immunol Methods 1994;172:209–217.
Harris LJ, Larson SB, Hasel KW, McPherson A: Refined structure of an intact IgG 2a monoclonal antibody. Biochemistry 1997;36:1581–1597.
Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antobe F, Ghetie V, Ward ES: The MHC Class I related receptor, FcR n, plays and essential role in the maternofetal transfer of gamma globulin in humans. Int Immunol 2001;13:993–1002.
Kristoffersen EK: Human placental Fc gamma-binding proteins in the maternofetal transfer of IgG. APMIS Suppl 1996;64:5–36.
Sondermann P, Huber R, Oosthuizen V, Jacob U: The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaR III complex. Nature 2000;406:267–273.
Hershko A, Ciechanover A: Mechanisms of intracellular protein breakdown. Annu Rev Biochem 1982;51:335–364.
Hershko A, Ciechanover A, Varshavsky A: The ubiquitin system. Nat Med 2000;6:1073–1081.
Dice JF: Molecular deteminants of protein half-lives in eukaryotic cells. FASEB J 1987;1:349–357.
Bachmair A, Finley D, Varshavsky A: In vivo half-life of a protein is a function of its amino-teminal residue. Science 1986;234:179–186.
Hershko A: Ubiquitin-mediated protein degradation. J Biol Chem 1988;263:15,237–15,240.
Contractor SF, Eaton BM, Stannard PJ: Uptake and fate of exogenous immunoglobulin G in the perfused human placenta. J Reprod Immunol 1983;5:265–273.
Wild A. E.: Endocyte mechanism in protein transfer across the placenta. Placenta Suppl 1981;1:165–173.
Rodewald R: Intestinal transport of peroxidase-conjugated IgG fragments in the neonatal rat. In: Hemmings WA (ed.): Maternofetal transmission of immunoglobulins. Cambridge: Cambridge University Press, 1976, pp. 137.
Parham P: On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol 1983;131:2895–2902.
Michaelsen TE: Fragmentation and conformational changes of IgG subclasses. In: Shakib F (ed.): The Human IgG subclasses: Molecular Aranysis, Structure, Function and Regulation. Oxford: Pergamon Press, 1990; pp. 31–41.
West APJ, Bjorkman PJ: Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry 2000;39:9698–9708.
Malek A, Sager R, Zakher A, Schneider H: Transport of immunoglobulin G and its subclasses across the in vitro-perfused human placenta. Am J Obstet Gynecol 1995;173:760–767.
Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL: Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol 1996;157:3317–3322.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghetie, V., Ward, E.S. Transcytosis and catabolism of antibody. Immunol Res 25, 97–113 (2002). https://doi.org/10.1385/IR:25:2:097
Issue Date:
DOI: https://doi.org/10.1385/IR:25:2:097