Summary
Background. The clinicopathological and biological significance of the expression of iNOS in pancreatic cancer remains unclear. The goal of this study was to determine the possible roles and clinical significance of iNOS expression in pancreatic cancer.
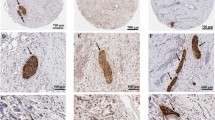
Methods. Seventy-two pancreatic adenocarcinoma tissue specimens were obtained by surgical resection. We investigated the immunohistochemical expression of iNOS in 72 patients with pancreatic cancer with respect to variable clinicopathological characteristics, proliferation activity (assessed by Ki-67 expression), apoptosis (assessed by TUNEL stain), and microvessel density (assessed by CD34 expression; angiogenesis).
Results. Immunohistochemical investigations demonstrated immunolabeling of tumor cells with anti-iNOS antibody. Positivity for iNOS was observed in 48/72 (66.7%). The expression of iNOS protein did not correlate with age, bilirubin, tumor marker, location, size, AJCC stage, differentiation, distant metastasis, or patient survival. No significanct association was found between iNOS expression and proliferation or microvessel density in pancreatic cancer. Apoptotic index (AI) of positive iNOS expressions were significantly higher than negative expression (p<0.001).
Conclusion. Inducible nitric oxide synthase (iNOS) is expressed by human pancreatic cancer, and its presence is positively correlated with apoptosis of cancer cells that could provide the basis for the development of therapeutic strategies in human pancreatic cancer.
Similar content being viewed by others
References
Rosewicz S, Wiedenmann B. Pancreatic carcinoma. Lancet 1997;349(9050):485–489.
Pancreas Club Meeting May 11, 1997 Bethesda, Maryland. Am J Surg 1998;175(3):172–178.
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002–2012.
Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 1994;298:249–258.
Cifone MG, Cironi L, Meccia MA, Roncaioli P, Festuccia C, De Nuntiis G, et al. Role of nitric oxide in cell-mediated tumor cytotoxicity. Adv Neuroimmunol 1995;5(4):443–461.
Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or-independent mechanisms. Cancer Res 1994;54(9):2462–2467.
Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer 1995;72:41–44.
Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Khan MA, et al. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer 1998;78(2):233–239.
Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer 1998;82:1897–1903.
Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res 1994;54:1352–1354.
Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res 1998;58:334–341.
Thomsen LL, Miles DW. Role of nitric oxide in tumor progression: lessons from human tumors. Cancer Metastasis Rev 1998;17:107–118.
Edwards P, Cendan JC, Topping DB, Moldawer LL, MacKay S, Copeland EM III, et al. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J Surg Res 1996;63(1):49–52.
Jadeski LC, Lala PK. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol 1999;155(4):1381–1390.
Xie K, Huang S, Dong Z, Juang SH, Gutman M, Xie QW, et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med 1995;181(4):1333–1343.
Nicotera P, Bonfoco E, Brune B. Mechanisms for nitric oxide-induced cell death: involvement of apoptosis. Adv Neuroimmunol 1995;5(4):411–420.
Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 1995;92(10):4392–4396.
Hajri A, Metzger E, Vallat F, Coffy S, Flatter E, Evrard S, et al. Role of nitric oxide in pancreatic tumour growth: in vivo and in vitro studies. Br J Cancer 1998;78(7):841–849.
Gansauge S, Nussler AK, Beger HG, Gansauge F. Nitric oxide-induced apoptosis in human pancreatic carcinoma cell lines is associated with a G1-arrest and an increase of the cyclin-dependent kinase inhibitor p21 WAF1/CIP1. Cell Growth Differ 1998;9(8):611–617.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA 1996;93(6):2442–2447.
Messmer UK, Brune B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem J 1996;319(Pt 1):299–305.
Xie K, Wang Y, Huang S, Xu L, Bielenberg D, Salas T, et al. Nitric oxide-mediated apoptosis of K-1735 melanoma cells is associated with downregulation of Bcl-2. Oncogene 1997;15(7):771–779.
Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Investig 1997;99(11):2625–2634.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, G., Kim, E.K., Kim, W.S. et al. Inducible nitric oxide synthase (iNOS) immunoreactivity and its relationship to cell proliferation, apoptosis, angiogenesis, clinicopathologic characteristics, and patient survival in pancreatic cancer. International Journal of Pancreatology 29, 133–140 (2001). https://doi.org/10.1385/IJGC:29:3:133
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/IJGC:29:3:133