Summary
Methods. Three-month-old female Wistar rats were fed with 20% alcohol in their drinking fluid over 6–17 mo using an interrupted feeding regimen. At different times, pancreatic acini were isolated by mild collagenase digestion. The concentrations of inositol-1,4,5-trisphosphate (1,4,5-IP3) were determined by a specific radioreceptor assay, before and at different times after stimulation with varying concentrations of CCK-8. CCK-induced dynamics of cytoplasmic calcium ([Ca2+]c) was investigated in acinar cells by confocal laser raster microscopy. Acinar α-amylase (Aml) secretion was measured as enzyme activity in the medium compared to the total activity in the suspension.
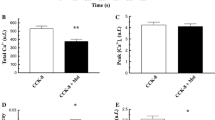
Results. In 12–13-mo-old rats, the CCK-stimulated 1,4,5-IP3 formation in acini was found to be decreased compared to young rats (age 4 mo). In rats of the same age fed with ethanol from the age of 3 mo on, 1,4,5-IP3 concentrations in acini were higher and reached values comparable to those in young rats. Corre-spondingly, the CCK-induced [Ca2+]c dynamics in acini isolated from 9-mo-old rats was impaired compared to that of young rats but normal in aged, chronically alcohol-fed rats. Aml secretion under CCK stimulation, however, which was decreased in aged rats, was additionally impaired after alcohol feeding.
Conclusion. Chronic alcohol feeding modifies 1,4,5-IP 3 formation, the [Ca2+]c dynamics of, and the Aml secretion of rat pancreatic acini in response to CCK stimulation. Obviously, the age-related impairment of 1,4,5-IP3 formation and [Ca2+]c dynamics is improved. In contrast, the decrease in Aml secretion of acini isolated from aged rats is more pronounced after long-term alcohol-feeding.
Similar content being viewed by others
References
Singer MV, Müller MK. Epidemiologie, Ätiologie und Pathogenese der chronischen Pankreatitis, in Erkrankungen des exkretorischen Pankreas, Mössner J, Adler G, Fölsch UR, Singer MV, eds., Gustav Fischer Verlag, Jena 1995; pp. 313–324.
Wilson JS, Korsten MA, Pirola RC. Alcohol-induced pancreatic injury (Part 2) Evolution of pathogenetic theories. Int J Pancreatol 1989; 4: 233–250.
Singh M. Alcoholic pancreatitis. Int J Pancreatol 1991; 8: 111–118.
Grönroos JM, Aho HJ, Nevalainen TJ. Cholinergic hypothesis of alcoholic pancreatitis. Dis Dis 1992; 10: 38–45.
Hoek JB, Thomas AP, Rooney TA, Higashi K, Rubin E. Ethanol and signal transduction in the liver. FASEB J 1992; 6: 2386–2396.
Saso K, Higashi K, Nomura T, Hoshino M, Ito M, Moehren G, Hoek JB. Inhibitory effect of ethanol on hepatocyte growth factor-induced DNA synthesis and Ca2+ mobilization in rat hepatocytes. Alcohol Clin Exp Res 1996; 20: 330A-334A.
Zhang B-H, Farrell GC. Ethanol perturbs receptor-operated cytosolic free calcium concentration signals in cultured rat hepatocytes. Gastroenterology 1997; 113: 641–648.
Bruzzone R. The molecular basis of enzyme secretion. Gastroenterology 1990; 99: 1157–1176.
Yule DI, Williams JA. Stimulus-secretion coupling in the pancreatic acinus, in Physiology of the Gastrointestinal Tract, Johnson LR, ed., Raven, New York, 1994; pp 1447–1472.
Habara Y, Kanno T. Stimulus-secretion coupling and Ca2+ dynamics in pancreatic acinar cells. Gen Pharmac 1994; 25: 843–850.
Rinderknecht H, Stace NH, Renner IG. Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Dig Dis Sci 1985; 30: 65–71.
Singh M. Modification by sex of diet and ethanol effect on rat pancreatic acinar cell metabolism. Pancreas 1986; 1: 164–171.
Siegmund E, Jonas L, Dummler W, Käding U, Kesting S. Zusammenhang zwischen Azinuszellverfettung und sekretorischer Kapazität des Rattenpankreas im Frühstadium einer alkoholinduzierten Pankreopathie. Z Gastroenterol 1992; 30: 385–390.
Tsukamoto H. Cellular pathophysiology of pancreatic acini during early stage of chronic alcoholic pancreatitis. Dig Dis Sci 1987; 32: 1190.
Grönroos JM, Kaila T, Aho HJ, Nevalainen TJ. Decrease in the number of muscarinic receptors in rat pancreas after chronic alcohol intake. Pharmacol Toxicol 1989; 64: 356–359.
Ponnappa BC, Hoek JB, Waring AJ, Rubin E. Effect of ethanol on amylase secretion and cellular calcium home-ostasis in pancreatic acini from normal and ethanol-fed rats. Biochem Pharmacol 1987; 36: 69–79.
Ponnappa BC, Dodge GR, Dudkowski B, Hoek JB, Iozzo RV, Rubin E. Stimulation of protein synthesis in isolated pancreatic acini from chronically ethanol-fed rats is due to alterations in post-transcriptional regulation. Biochim Biophys Acta 1993; 1158: 113–119.
Schmidt DN, Pandol SJ. Differing effects of ethanol on in vitro stimulated pancreatic enzyme secretion in ethanolfed and control rats. Pancreas 1990; 5: 27–36.
Siegmund E, Jonas L, Borisch I, Fechner U, Käding U, Schröder H. Different dosages of acetylsalicylic acid lead to adverse modifications of the reaction of rat pancreas to ethanol. Int J Pancreatol 1998; 23: 125–136.
Rick W, Stegbauer HP. α-Amylase. Messung der reduzierenden Gruppen, in Methoden der enzymatischen Analyse, Bergmeyer HU, ed., Verlag Chemie, Weinheim 1974; pp. 918–923.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275.
Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 1980; 102: 344–352.
Stephens MA. EDF statistics for goodness of fit and some comparisons. J Am Stat Assoc 1974; 69: 730–737.
Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 1993; 361: 315–325.
Merritt JE, Taylor CW, Rubin RP, Putney JW. Isomers of inositol trisphosphate in exocrine pancreas. Biochem J 1986; 238: 825–829.
Rutherford RE, Schoeffield-Payne M, Pandol SJ. Cellular mechanisms and physiological implications of quantal Ca2+ release in pancreatic acinar cells. Am J Physiol 1994; 267: G1058-G1066.
Matozaki T, Williams JA. Multiple sources of 1,2-diacylglycerol in isolated rat pancreatic acini stimulated by cholecystokinin. J Biol Chem 1989; 264: 14,729–14,734.
Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang L-H, Martinez J, Gardner JD, Jensen RT. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol 1990; 259: G655-G665.
Stryjek-Kaminska D, Piiper A, Stein J, Caspary WF, Zeuzem S. Epidermal growth factor receptor signaling in rat pancreatic acinar cells. Pancreas 1995; 10: 274–280.
Trimble ER, Bruzzone R, Meehan CJ, Biden TJ. Rapid increases in inositol 1,4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate and eytosolic free Ca2+ in agonist-stimulated pancreatic acini of the rat. Biochem J 1987; 242: 289–292.
Dixon JF, Hokin LE. Inositol 1,2-cyclic 4,5-trisphosphate concentration relative to inositol 1,4,5-trisphosphate in pancreatic minilobules on stimulation with carbamylcholine in the absence of lithium. J Biol Chem 1987; 262: 13,892–13,895.
Lipschitz DA, Udupa KB, Indelicato SR, Das M. Effect of age on second messenger generation in neutrophils. Blood 1991; 78: 1347–1354.
Fulop T, Barabas G, Varga Z, József C, Csabina S, Szucs S, Seres I, Szikszay E, Jeney Z, Penyige A. Age-dependent changes in transmembrane signalling: identification of G proteins in human lymphocytes and polymorphonuclear leukocytes. Cell Signal 1993; 5: 593–603.
Utsuyama M, Wakikawa A, Tamura T, Nariuchi H, Hirokawa K. Impairment of signal transduction in T cells from old mice. Mech Ageing Dev 1997; 93: 131–144.
Igwe O, Filla MB. Aging-related regulation of myoinositol 1,4,5-trisphosphate signal transduction pathway in the rat striatum. Mol Brain Res 1997; 46: 39–53.
de Boland AR, Facchinetti MM, Balogh G, Massheimer V, Boland RL. Age-associated decrease in inositol 1,4,5-trisphosphate and diacylglycerol generation by 1,25(OH)2-Vitamin D3 in rat intestine. Cell Signal 1996; 8: 153–157.
Roth GS. Mechanisms of altered hormone-neurotransmitter action during aging: from receptors to calcium mobilization. Ann Rev Gerontol Geriatr 1990; 10: 132–146.
Seagrave J, Hildebrand R, Johnson LJ. Muscarinic signalling in submandibular salivary acinar cells of aging rats. Archs Oral Biol 1996; 41: 425–430.
Olson JG, Salih MA, Harrison JL, Herrera I, Luther MF, Kalu DN, Lifschitz MD, Katz MS, Yeh C-K. Modulation by food restriction of intracellular calcium signaling in parotid acinar cells of aging Fischer 344 rats. J Gerontol: Biol Sci 1997; 52A: B152-B158.
Majumdar APN, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc Soc Exp Biol Med 1997; 215: 134–144.
Mironov SL, Hermann A. Ethanol actions on the mechanisms of Ca2+ mobilization in rat hippocampal cells are mediated by protein kinase C. Brain Res 1996; 714: 27–37.
Lahnsteiner E, Hermann A. Acute action of ethanol on rat hippocampal CA1 neurons: effects on intracellular signaling. Neurosc Letts 1995; 19: 153–156.
Gaisano HY, Miller LJ, Foskett JK. Suppression of Ca2+ oscillations induced by cholecystokinin (CCK) and its analog OPE in rat pancreatic acinar cells by low-level protein kinase C activation without transition of the CCK receptor from a high- to low-affinity state. Pflügers Arch 1994; 427: 455–462.
Sung CK, Hootman SR, Stuenkel EL, Kuroiwa C, Williams JA. Downregulation of protein kinase C in guinea pig pancreatic acini: effects on secretion. Am J Physiol 1988; 254: G242-G248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siegmund, E., Pommerenke, H., Jonas, L. et al. Inositol 1,4,5-trisphosphate formation, cytoplasmic calcium dynamics, and α-amylase secretion of pancreatic acini isolated from aged and chronically alcohol-fed rats. International Journal of Pancreatology 27, 39–50 (2000). https://doi.org/10.1385/IJGC:27:1:39
Issue Date:
DOI: https://doi.org/10.1385/IJGC:27:1:39