Summary
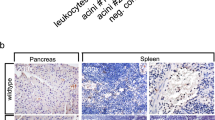
Background. Phospholipase A2 (PLA2) may play a central role in the pathogenesis of pancreatic acinar cell necrosis. Several questions, however, are unsolved: Is acinar cell necrosis caused by PLA2 derived from infiltrating leukocytes or from pancreatic PLA2 itself? Does PLA2 cause cellular lysis by the release of lysolecithin from lecithin or by generation of free radicals? The aims of this study were to determine which form of PLA2 is responsible for cellular damage and how to inhibit its action.
Methods. Isolated rat pancreatic acini were prepared by collagenase digestion. Newly synthesized proteins were labeled by 35S-methionine. Acini were incubated in buffer to which various factors, such as porcine pancreatic PLA2 or bee venom PLA2, homogenates of either leukocytes or pancreatic homogenates, all with or without lecithin and with or without potential inhibitors (aprotinin, 4-bromophenacylbromide, BM 16.2115, quinacrine, various analogs of arachidonic acid), or free radicals (hydrogen peroxide, xanthine/xanthine oxidase) with or without allo-purinol or dismutase/catalase were added. Cellular destruction was measured by the release of radiolabeled proteins.
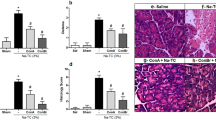
Results. PLA2 alone, free radicals, and granulocytes were not harmful to acini within 30 min of incubation. Free radicals caused significant release of radiolabeled proteins only after 3 h of incubation; this release could be inhibited by scavengers. Incubation of pancreatic acini with PLA2 in combination with lecithin caused rapid release of radiolabeled proteins. Addition of high concentrations of enterokinase activated pancreatic homogenates both alone and with lecithin caused release of cellular proteins, suggesting that pancreatic PLA2 uses lecithin from pancreatic membranes as substrate. Almost all tested potential inhibitors of PLA2 were unable to prevent the destruction caused by either pancreatic or bee venom PLA2 and lecithin. However, HK 42, a polyunsaturated fatty acid analog, was able to reduce dose dependently the release of acinar proteins caused by pancreatic PLA2 and lecithin.
Conclusion. Pancreatic PLA2 and not PLA2 from infiltrating leukocytes may play a role in pancreatic acinar cell necrosis. Cellular lysis is caused upon the action of lysolecithin and probably not via the action of free radicals.
Similar content being viewed by others
Abbreviations
- HR:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-Ringer
- Tris:
-
trometamol
- TCA:
-
trichloroacetic acid
- DMSO:
-
dimethylsulfoxide
- PLA2:
-
phospholipase A2
References
Mössner, J, Wessig C. Pancreatic phospholipase A2 (PLA2) may play a major role in the pathogenesis of experimental pancreatitis. Gastroenterology 1995; 108: A 377.
Creutzfeldt W, Schmidt H. Aetiology and pathogenesis of pancreatitis. Scand J Gastroenterol 1970; 5(Suppl 6): 47–62.
Malagelada J-R. The pathophysiology of alcoholic pancreatitis. Pancreas 1986; 1: 270–278.
Harvey MH, Cates MC, Reber HA. Possible mechanisms of acute pancreatitis induced by ethanol. Am J Surg 1988; 155: 49–56.
Singh M, Simsek H. Ethanol and the pancreas. Gastroenterology 1990; 98: 1051–1062.
Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med 1974; 290: 484–487.
Acosta JM, Pellegrini CA, Skinner DB. Etiology and pathogenesis of acute biliary pancreatitis. Surgery 1980; 88: 118–125.
Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 1985; 88: 1192–1204.
Powers RE, Saluja AK, Houlihan MJ, Steer ML. Diminished agonist-stimulated inositol trisphosphate generation blocks stimulus-secretion coupling in mouse pancreatic acini during diet-induced experimental pancreatitis. J Clin Invest 1986; 77: 1668–1674.
Steer ML, Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med 1987; 316: 144–150.
Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 1997; 113: 304–310.
Mithofer K, Fernandez-del Castillo C, Rattner D, Warshaw AL. Subcellular kinetics of early trypsinogen activation in acute rodent pancreatitis. Am J Physiol 1998; 274: G71-G79.
Lankisch PG, Ihse I. Bile-induced acute experimental pancreatitis. Scand J Gastroenterol 1987; 22: 257–260.
Rutledge PL, Saluja AK, Powers RE, Steer ML. Role of oxygen-derived free radicals in diet-induced hemorrhagic pancreatitis in mice. Gatroenterology 1987; 93: 41–47.
Sanfey H, Bulkley GB, Cameron JL. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg 1985; 201: 633–639.
Ito T, Nakao A, Kishimoto W, Nakano M, Takagi H. The involvement and sources of active oxygen in experimentally induced acute pancreatitis. Pancreas 1996; 12: 173–177.
Kuroda T, Shiohara E, Homma T, Furukawa Y, Chiba S. Effects of leukocyte and platelet depletion on ischemia-reperfusion injury to dog pancreas. Gastroenterology 1994; 107: 1125–1134.
Schmidt H, Creutzfeldt W. The possible role of phospholipase A in the pathogenesis of acute pancreatitis. Scand J Gastroenterol 1969; 4: 39–48.
Nevalainen TJ. The role of phospholipase A2 in acute pancreatitis. Scand J Gastroenterol 1980; 15: 641–650.
Terry TR, Herman-Taylor J, Grant DAW. The generation of lysolecithin by enterokinase in trypsinogen prophospholipase A2 lecithin mixtures, and its relevance to the pathogenesis of acute necrotising pancreatitis. Clin Chim Acta 1985; 150: 151–163.
Odaira C, Berger Z, Iovanna JL, Sarles H. Localized necro-hemorrhagic pancreatitis in the rat after pancreatic interstitial trypsin injection. Regressive pseudochronic lesions. Digestion 1986; 34: 68–77.
Nakae Y, Naruse S, Kitagawa M, Hirao S, Yamamoto R, Hayakawa T. Activation of trypsinogen in experimental models of acute pancreatitis in rats. Pancreas 1995; 10: 306–313.
Frick TW, Fernandez-del Castillo C, Bimmler D, Warshaw AL. Elevated calcium and activation of trypsinogen in rat pancreatic acini. Gut 1997; 41: 339–343.
Largman C, Reidelberger RD, Tsukamoto H. Correlation of trypsin-plasma inhibitor complexes with mortality in experimental pancreatitis in rats. Dig Dis Sci 1986; 31: 961–969.
Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol 1995; 38: C1295–1304.
Saluja A, Hofbauer B, Yamaguchi Y, Yamanaka K, Steer M. Induction of apoptosis reduces the severity of caerulein-induced pancreatitis in mice. Biochem Biophys Res Comm 1996; 200: 875–878.
Nagai H, Henrich H, Wünsch PH, Fischbach W, Mössner J. Role of pancreatic enzymes and their substrates in auto-digestion of the pancreas. In-vitro-studies with isolated rat pancreatic acini. Gastroenterology 1989; 86: 838–847.
Kimura W, Secknus R, Fischbach WC, Mössner J. Role of phospholipase A2 in pancreatic acinar cell damage and possibilities of inhibition. Studies with isolated rat pancreatic acini. Pancreas 1992; 8: 70–79.
Mössner J, Bödeker H, Kimura W, Meyer F, Böhm S, Fischbach W. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int J Pancreatol 1992; 12: 285–296.
Kimura W, Meyer F, Hess D, Kirchner Th, Fischbach W, Mössner J. Comparison of different treatment modalities in experimental pancreatitis: inhibition of proteases, inhibition of lipase, albumin. Gastroenterology 1992; 103: 1916–1924.
Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol 1978; 235: E517-E524.
Hoffmann GE, Schmidt D, Bastian B, Guder WG. Photometric determination of phospholipase A. J Clin Chem Clin Biochem 1986; 24: 871–875.
Erlanger BF, Kokowsky N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 1961; 95: 271–278.
Meyer F, Kimura W, Marczok V, Pusch B, Fischbach W, Mössner J. Stimulus secretion coupling and regeneration in various animal models of acute pancreatitis. Eur J Gastroenterol Hepatol 1993; 5: 275–282.
Kimura W, Mössner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol 1996; 20: 177–184.
Yuan W, Quinn DM, Sigler PB, Gelb MH. Kinetic and inhibition studies of phospholipase A2 with short-chain substrates and inhibitors. Biochemistry 1990; 29: 6082–6094.
Bayburt T, Yu BZ, Lin HK, Browning J, Jain MK, Gelb MH. Human nonpancreatic secreted phospholipase A2: interfacial parameters, substrate specificities, and competitive inhibitors. Biochemistry 1993; 32: 573–582.
Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry 1993; 32: 5935–5940.
Gelb MH, Jain MK, Berg OG. Inhibition of phospholipase A2. FASEB J 1994; 8: 916–924.
Gelb MH, Jain MK, Hanel AM, Berg OG. Interfacial enzymology of glycerolipid hydrolases: lessons from secreted phospholipases A2. Annu Rev Biochem 1995; 64: 653–688.
Bayburt T, Gelb MH. Interfacial catalysis by human 85 kDa cytosolic phospholipase A2 on anionic vesicles in the scooting mode. Biochemistry 1997; 36: 3216–3231.
Nevalainen TJ, Gronroos JM, Kortesuo PT. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut 1993; 34: 1133–1136.
Hiertaranta AJ, Peuravuori HJ, Nevalainen TJ. Phospholipase A2 in sodium taurocholate-induced experimental hemorrhagic pancreatitis in the rat. J Surg Res 1995; 59: 271–278.
Hietaranta A, Kemppainen E, Puolakkainen P, Sainio V, Haapiainen R, Peuravuori H, Kivilaakso E, Nevalainen T. Extracellular phospholipases A2 in relation to systemic inflammatory response syndrome (SIRS) and systemic complications in severe acute pancreatitis. Pancreas 1999; 18: 385–391.
Furue S, Hori Y, Kuwabara K, Ikeuchi J, Onoyama H, Yamamoto M, Tanaka K. Increased activity of group II phospholipase A2 in plasma in rat sodium deoxycholate induced acute pancreatitis. Gut 1997; 41: 826–831.
Yoshikawa T, Naruse S, Kitagawa M, Ishiguro H, Nakae Y, Ono T, Hayakawa T. Effect of a new inhibitor of type II phospholipase A2 on experimental acute pancreatitis in rats. Pancreas 1999; 19: 193–198.
Ito T, Nakao A, Kishimoto W, Nakano M, Takagi H. The involvement and sources of active oxygen in experimentally induced acute pancreatitis. Pancreas 1996; 12: 173–177.
Niederau C, Schulz H-U, Klonowski H. Lazaroids protect isolated pancreatic acinar cells against damage induced by free radicals. Pancreas 1995; 11: 107–121.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mössner, J., Wessig, C., Ogami, Y. et al. Role of various phospholipases A2 and inhibitors in the pathogenesis and prevention of pancreatic acinar cell necrosis. International Journal of Pancreatology 27, 29–38 (2000). https://doi.org/10.1385/IJGC:27:1:29
Issue Date:
DOI: https://doi.org/10.1385/IJGC:27:1:29