Abstract
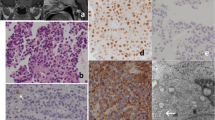
The differentiation of pituitary cells and human pituitary adenomas follow three cell lineages: H-PRTSH, ACTH, and FSHH, which are regulated by a combination of various transcription factors and co-factors. We have used R-PCRand immunohistochemistry to show that immunonegative, null cell″adenomas are equipped with multiple transcription factors and co-factors. The null cell″adenomas showed similar frequencies of transcription factors as did the gonadotropin subunit (GSU)positive adenomas, with the exception that there were fewer instances of SF1 in the former. We speculate, therefore, that null cell adenomas and GSUpositive adenomas share common molecular mechanisms in functional differentiation, even though the former do not produce hormones. From the high frequency of various transcription factors, we also speculate that both null cell adenomas and GSUpositive adenomas are derived from committed″pituitary progenitor stem cells. The questions, why a certain proportion of these pituitary tumor groups lack hormone production and why they are molecularly more committed to G transcription, remain to be further investigated.
Similar content being viewed by others
References
Andersen B, Rosenfeld MG. Pit-1 determines cell types during development of the anterior pituitary gland. A model for transcriptional regulation of cell phenotypes in mammalian organogenesis. J Biol Chem 269:29335–29338, 1994.
Asa SL, Bamberger AM, Cao B, Wong M, Parker KL, Ezzat S. The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab 81:2165–2170, 1996.
Aylwin SJ, Welch JP, Davey CL, et al. The relationship between steroidogenic factor 1 and DAX-1 expression and in vitro gonadotropin secretion in human pituitary adenomas. J Clin Endocrinol Metab 86:2476–2483, 2001.
Dasen JS, O’Connell SM, Flynn SE, et al. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97:587–598, 1999.
Gordon DF, Woodmansee WW, Black JN, et al. Domains of Pit-1 required for transcriptional synergy with GATA-2 on the TSH beta gene. Mol Cell Endocrinol 196:53–66, 2002.
Guy JC, Hunter CS, Showalter AD, et al. Conserved amino acid sequences confer nuclear localization upon the Prophet of Pit-1 pituitary transcription factor protein. Gene 336:263–273, 2004.
Haisenleder DJ, Yasin M, Dalkin AC, Gilrain J, Marshall JC. GnRH regulates steroidogenic factor-1 (SF-1) gene expression in the rat pituitary. Endocrinology 137:5719–5722, 1996.
Horvath E, Kovacs K. Pituitary gland. Pathol Res Pract 183:129–142, 1988.
Ikuyama S, Mu YM, Ohe K, et al. Expression of an orphan nuclear receptor DAX-1 in human pituitary adenomas. Clin Endocrinol (Oxf) 48:647–654, 1998.
Ikuyama S, Ohe K, Sakai Y, et al. Follicle stimulating hormone-beta subunit gene is expressed in parallel with a transcription factor Ad4BP/SF-1 in human pituitary adenomas. Clin Endocrinol (Oxf) 45:187–193, 1996.
Kovacs K, Horvath E, Ryan N, Ezrin C. Null cell adenoma of the human pituitary. Virchows Arch A Pathol Anat Histol 387:165–174, 1980.
Lamolet B, Pulichino AM, Lamonerie T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859, 2001.
Lloyd RV, Osamura RY. Transcription factors in normal and neoplastic pituitary tissues. Microsc Res Tech 39:168–181, 1997.
Nakamura S, Ohtsuru A, Takamura N, et al. Prop-1 gene expression in human pituitary tumors. J Clin Endocrinol Metab 84:2581–2584, 1999.
Nakamura Y, Usui T, Mizuta H, et al. Characterization of Prophet of Pit-1 gene expression in normal pituitary and pituitary adenomas in humans. J Clin Endocrinol Metab 84:1414–1419, 1999.
Nogami H, Inoue K, Moriya H, et al. Regulation of growth hormone-releasing hormone receptor messenger ribonucleic acid expression by glucocorticoids in MtT-S cells and in the pituitary gland of fetal rats. Endocrinology 140:2763–2770, 1999.
Osamura RY, Tahara S, Komatsubara K, et al. Pit-1 positive alpha-subunit positive nonfunctioning human pituitary adenomas: a dedifferentiated GH cell lineage? Pituitary 1:269–271, 1999.
Osamura RY, Watanabe K. Immunohistochemical studies of human FSH producing pituitary adenomas. Virchows Arch A Pathol Anat Histopathol 413:61–68, 1988.
Oyama K, Sanno N, Teramoto A, Osamura RY. Expression of neuro D1 in human normal pituitaries and pituitary adenomas. Mod Pathol 14:892–899, 2001.
Palomino T, Sanchez Pacheco A, Pena P, Aranda A. A direct protein-protein interaction is involved in the cooperation between thyroid hormone and retinoic acid receptors and the transcription factor GHF-1. FASEB J 12:1201–1209, 1998.
Pulichino AM, Vallette Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747, 2003.
Sanno N, Jin L, Qian X, et al. Gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor messenger ribonucleic acids expression in nontumorous and neoplastic pituitaries. J Clin Endocrinol Metab 82:1974–1982, 1997.
Sanno N, Teramoto A, Matsuno A, Osamura RY. Expression of human Pit-1 product in the human pituitary and pituitary adenomas. Immunohistochemical studies using an antibody against synthetic human Pit-1 product. Arch Pathol Lab Med 120:73–77, 1996.
Sanno N, Teramoto A, Sugiyama M, et al. Expression of Pit-1 mRNA and activin/inhibin subunits in clinically nonfunctioning pituitary adenomas. In situ hybridization and immunohistochemical analysis. Horm Res 50:11–17, 1998.
Sheng HZ, Moriyama K, Yamashita T, et al. Multistep control of pituitary organogenesis. Science 278:1809–1812, 1997.
Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 129:329–337, 2002.
Tahara S, Kurotani R, Sanno N, et al. Expression of pituitary homeo box 1 (Ptx1) in human non-neoplastic pituitaries and pituitary adenomas. Mod Pathol 13:1097–1108, 2000.
Tremblay JJ, Goodyer CG, Drouin J. Transcriptional properties of Ptx1 and Ptx2 isoforms. Neuroendocrinology 71:277–286, 2000.
Tremblay JJ, Lanctot C, Drouin J. The panpituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441, 1998.
Umeoka K, Sanno N, Osamura RY, Teramoto A. Expression of GATA-2 in human pituitary adenomas. Mod Pathol 15:11–17, 2002.
Vallette Kasic S, Figarella Branger D, Grino M, et al. Differential regulation of proopiomelanocortin and pituitary-restricted transcription factor (TPIT), a new marker of normal and adenomatous human corticotrophs. J Clin Endocrinol Metab 88:3050–3056, 2003.
West BE, Parker GE, Savage JJ, et al. Regulation of the follicle-stimulating hormone beta gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology 145:4866–4879, 2004.
Zhao D, Tomono Y, Tsuboi K, Nose T. Immunohistochemical and ultrastructural study of clinically nonfunctioning pituitary adenomas. Neurol Med Chir (Tokyo) 40:453–456, discussion 456–457, 2000.
DeLellis RA, Lloyd RV, Heitz PU, Eng C. WHO classification of tumors. Pathology and genetics of tumors of endocrine organs. Lyon, France: IARC, 2004.
Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene 319:1–19, 2003.
Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science 295:2231–2235, 2002.
Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws ER Jr. Selective expression of estrogen receptor alpha and beta isoforms in human pituitary tumors. J Clin Endocrinol Metab 83:3965–3972, 1998.
Asa SL, Puy LA, Lew AM, Sundmark VC, Elsholts HP. Cell type-specific expression of the pituitary transcription activator pit-1 in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab 77:1275–1280, 1993.
Simmons DM, Voss JW, Ingraham HA, et al. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4(5):695–711, 1990.
Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7(7):852–860, 1993.
Morohashi K, Iida H, Nomura M, et al. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol 8(5):643–653, 1994.
Naya FJ, Stellrecht CM, Tsai Mj. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev 9(8):1009–1019, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishii, Y., Suzuki, M., Takekoshi, S. et al. Immunonegative null cell″adenomas and Gnadotropin (G) Subunit (Su) immunopositive adenomas share frequent expression of multiple transcription factors. Endocr Pathol 17, 35–44 (2006). https://doi.org/10.1385/EP:17:1:35
Issue Date:
DOI: https://doi.org/10.1385/EP:17:1:35