Abstract
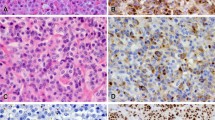
Dopamine (DA) agonists cause reduction of blood prolactin level and tumor shrinkage in most patients with lactotroph adenoma. Our aim was to investigate the cellular mechanism of tumor shrinkage by determining mitotic, MIB-1, p27, and apoptotic indices, as well as microvessel density (MVD), surface microvessel density (SMD), ploidy, and other nuclear parameters. Surgically removed lactotroph adenomas were selected from 29 patients, of whom 19 were treated with oral bromocriptine (BEC), long-acting injectable BEC (BEC-LAR), or quinagolide and 10 were untreated. In treated adenomas mitotic and MIB-1 indices were lower, whereas the apoptotic indices were not significantly higher compared to untreated adenomas. The decrease in MIB-1 labeling reached significance in adenomas exposed to quinagolide (p<0.05). Aside from the BEC-LAR treated group, wherein p27 expression was significantly reduced (p<0.05), p27 expression did not differ significantly between the treated and untreated groups. MVD density was significantly lower in the treated adenomas, whereas the decrease in SMD did not attain significance. The DNA ploidy and most other nuclear parameters did not differ significantly in the two groups. In conclusion, reduction of mitotic and MIB-1 indices indicates that suppression of cell proliferation contributes to tumor shrinkage, whereas p27 protein expression and apoptosis play no major role in the adenoma involution. Further studies are required to explain the effect of DA agonists on MVD and SMD.
Similar content being viewed by others
References
Bevan JS, Webster J, Burke CW, Scanlon MF. Dopamine agonists and pituitary tumor shrinkage. Endocr Rev 13:220–240, 1992.
Ciccarelli E, Miola C, Grottoli S, Avataneo T, Lancranjan I, Camanni F. Long term therapy of patients with macroprolactinoma using repeatable injectable bromocriptine. J Clin Endocrinol Metab 76:484–488, 1993.
Tabarin A, Catargi B. Treatment of macroprolactinomas with quinagolide (Norprolac). Annu d’Endocrinol 58:87–94, 1997.
Lloyd HM, Meares JD, Jacobi J. Effects of oestrogen and bromocriptine on in vivo secretion and mitosis in prolactin cells. Nature 255:497–498, 1975.
Tindall GT, Kovacs K, Horvath E, Thorner MO. Human prolactin-producing adenomas and bromocriptine: a histological, immunocytochemical, ultrastructural and morphometric study. J Clin Endocrinol Metab 55:1178–1183, 1982.
Wood DF, Johnston JM, Johnston DG. Dopamine, the dopamine D2 receptor and pituitary tumours. Clin Endocrinol 35:455–466, 1991.
Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology 17:489–503, 1990.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715, 1984.
Ekramullah SM, Saitoh Y, Arita N, Ohnishi T, Hayakawa T. The correlation of Ki-67 staining indices with tumour doubling times in regrowing non-functioning pituitary adenomas. Acta Neurochir (Wein) 138:1449–1455, 1996.
Losa M, Franzin A, Mortini P, Terreni MR, Mangili F, Giovanelli M. Usefulness of markers of cell proliferation in the management of pituitary adenomas. Clin Sci 95:29–135, 1998.
Sallinen PK, Haapasalo HK, Visakoripi T, Helen PT, Rantala IS, Isola JJ, Helin HJ. Prognostification of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S phase fraction using archival paraffin-embedded samples. J Pathol 174:275–282, 1994.
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38:99–106, 1996.
Thor AD, Liu S, Moore DH, Edgerton SM. Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol 17:470–471, 1999.
Sherr CJ. Cancer cell cycles. Science 274:1672–1677, 1996.
Kiyokawa H, Kineman RD, Manova-Todorova KO, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27kip1. Cell 85:721–732, 1996.
Fero ML, Rivkin M, Tasch M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27kip1-deficient mice. Cell 85:733–744, 1996.
Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707–720, 1996.
Lloyd RV, Erickson LA, Jin L, et al. P27kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154:313–323, 1999.
Newcomb EW, Sosnow M, Demopoulos RI, Zeleniuch-Jacquotte A, Sorich J, Speyer JL. Expression of the cell cycle inhibitor p27kip1 is a new prognostic marker associated with survival in epithelial ovarian tumors. Am J Pathol 154:119–125, 1999.
Saegusa M, Nitta H, Hashimura M, Okayasu I. Down-regulation of p27kip1 expression is correlated with increased cell proliferation but not expression of p21 waf1 and p53 and human papillomavirus infection in benign and malignant tumours of sinonasal regions. Histopathology 35:55–64, 1999.
Erickson LA, Jin L, Wollen P, Thompson GB, van Heerden JA, Lloyd RV. Parathyroid hyperplasia, adenomas, and carcinomas differential expression of p27kip1 protein. Am J Surg Pathol 23:288–295, 1999.
Nakzumi H, Sasano H, Iino K, Ohashi Y, Orikasa S. Expression of cell cycle inhibitor p27 and Ki-67 in human adrenocortical neoplasms. Mod Pathol 11:1165–1170, 1998.
Reed JC, Miyashita T, Takayama S, et al. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 60:23–32, 1996.
Kontogeorgos G, Sambaziotis D, Piaditis G, Karameris A. Apoptosis in human pituitary adenomas: a morphological and in-situ end-labeling study. Mod Pathol 10:921–926, 1997.
Kulig E, Jin L, Xiang Q, et al. Apoptosis in nontumorous and neoplastic human pituitaries. Expression of the BCL-2 family of proteins. Am J Pathol 154:767–774, 1999.
Green VL, White MC, Hipkin LJ, Jeffreys RV, Foy PM, Atkin SL. Apoptosis and p53 suppressor gene protein expression in human anterior pituitary adenomas. Eur J Endocrinol 136:382–387, 1997.
Folkman J, What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6, 1990.
Monschke F, Muller WU, Winkler U, Steffer C. Cell proliferation and vascularization in human breast carcinomas. Int J Cancer 49:812–815, 1991.
Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol 10:302–313, 1993.
Barnhill RL, Fandrey K, Levy MA, Mihm MC, Hyman B. Angiogenesis and tumor progression of melanoma. Quantification of vascularity in melanocytic nevi and cutaneous malignant melanoma. Lab Invest 67:331–337, 1992.
Dinh TV, Hannigan EV, Smith ER, Hove MJ, Chopra V, To T. Tumor angiogenesis as a predictor of recurrence in stage lb squamous cell carcinoma of the cervix. Obst Gynecol 87:751–754, 1996.
Jugenburg M, Kovacs K, Jugenburg I, Scheithauer BW. Angiogenesis in endocrine neoplasms. Endocr Pathol 8:259–272, 1997.
Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85:1159–1162, 2000.
Jakubowski J. Blood Supply, blood flow and autoregulation in the adenohypophysis, and altered patterns in oestrogen-induced adenomatous hyperplasia. Br J Neurosurg 9:331–345, 1995.
Pressman N J. Markovian analysis of cervical cell images. J Histochem Cytochem 24:138–144, 1976.
Rengachary SS, Tomita T, Jefferies B, Watanabe I. Structural changes in human pituitary tumor after bromocriptine therapy. Neurosurgery 10:242–251, 1982.
Gen M, Uozumi T, Ohta M, Ito A, Kajiwara H, Mori S. Necrotic changes in prolactinomas after long term administration of bromocriptine. J Clin Endocrinol Metab 59:463–479, 1983.
Ekramullah SM, Saitoh Y, Ohnishi T, Arita N, Taki T, Hayakawa T. Effects of bromocriptine on staining indices of Ki-67 and proliferating cell nuclear antigen and nucleolar organizer region number in pituitary adenomas. Neurol Med Chir 35:221–226, 1940.
Burger PC, Shibata T, Kleihues P. Proliferation markers for neoplasms of the nervous system. In: Advances in Immunohistochemistry, DeLellis RA (ed), New York, Raven Press, 1988; 302–316.
Thapar K, Yamada Y, Scheithauer BW, Kovacs K, Yamada S, Stefaneanu L. Assessment of mitotic activity in pituitary adenomas and carcinomas. Endocr Pathol 7:215–221, 1996.
Shibuya M, Saito F, Miwa T, Davis RL, Wilson CB, Hoshino T. Histochemical study of pituitary adenomas with Ki-67 and anti-DNA polymerase a monoclonal antibodies, bromo-deoxyuridine labeling, and nucleolar organizer region counts. Acta Neuropathol 84:178–183, 1992.
Stefaneanu L, Kovacs K, Horvath E, et al. In-situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab 78:83–88, 1993.
Lloyd RV, Cano M, Landerfeld TD. The effects of estrogens on tumor growth and on prolactin and growth hormone mRNA expression in rat pituitary tissues. Am J Pathol 133:397–406, 1988.
Waterman ML, Adler S, Nelson C, Greene GL, Evans R, Rosenfeld MG. A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol Endocrinol 2:14–21, 1988.
Bamberger CM, Fehn M, Bamberger AM, et al. Reduced expression levels of the cell-cycle inhibitor p27 in human pituitary adenomas. Eur J Endocrinol 140:250–255, 1999.
Jin L, Qian X, Kulig E, et al. Transforming growth factor-β, transforming growth factor-β receptor II, and p27kip1 expression in nontumorous and neoplastic human pituitaries. Am J Pathol 151:509–519, 1997.
Tanaka C, Yoshimoto K, Yang P, et al. Infrequent mutations of p27kip1 gene and trisomy 12 in a subset of human pituitary adenomas. J Clin Endocrinol Metab 82:3141–3147, 1997.
Yin D, Kondo S, Takeuchi J, Morimura T. Induction of apoptosis in murine ACTH-secreting pituitary adenoma cells by bromocriptine. FEBS Lett 339:73–75, 1994.
Drewett N, Jacobi JM, Willgoss DA, Lloyd HM. Apoptosis in the anterior pituitary gland of the rat: studies with estrogen and bromocriptine. Neuroendocrinology 57:89–95, 1993.
Weidner N, Semple JP, Welch WR, Folkman JF. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med 324:1–8, 1991.
Bostwick DG, Wheeler TM, Blute M, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology 48:47–57, 1996.
Bodmer CW, Atkin SL, Savage MW, Masson EA, White MC. Effects of quinagolide (CV205-502), a selective D2-agonist, on vascular reactivity in patients with prolactin-secreting adenoma. Clin Endocrinol 43:49–53, 1995.
Kemeny AA, Jakubowski JA, Pasztor Z, Jefferson AA, Wojcikiewicz R. Reduction of blood flow in the adenohypophysis of rats by bromocriptine. J Neurosurg 63:120–124, 1985.
Kemeny AA, Jakubowski J, Stawowy A, Smith C, Timperley WR. Changes of blood flow in oestrogen induce hyperplastic anterior lobe following bromocriptine administration. Br J Neurosurg 1:243–250, 1987.
Kobayashi Y, Amenta F, Ricci A, Hattori K. Localisation of dopamine D1-like and D2-like receptors in the pulmonary vasculature. Hypertension Res 1:S153–156, 1995.
Kobayashi Y, Cavallotti D, Ricci A, Amenta F. Localisation of dopamine D2-like receptors in pulmonary artery of the human and rabbit but not of the rat. Eur J Pharmacol 261:229–236, 1994.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefaneanu, L., Kovacs, K., Scheithauer, B.W. et al. Effect of dopamine agonists on lactotroph adenomas of the human pituitary. Endocr Pathol 11, 341–352 (2000). https://doi.org/10.1385/EP:11:4:341
Issue Date:
DOI: https://doi.org/10.1385/EP:11:4:341