Abstract
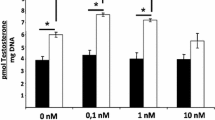
The mechanism of arginine vasopressin (AVP) action in Leydig cells was investigated, and compared to the effects of phorbol-13-myristate-12-acetate (PMA) and interleukin-1β (IL-1β). Previous reports suggested that AVP inhibits Leydig cell testosterone production at the level of 17α-hydroxylase/C17-lyase activity. The present study confirms and extends these observations, and contrasts the effects of AVP to IL-1. In all experiments, macrophage-depleted Leydig cells were isolated from mice and maintained in primary culture for 5 d prior to initiation of treatments. Leydig cells were treated with 8-Br-cAMP plus increasing concentrations of AVP or IL-1β. AVP caused a significant and dose-dependent inhibition of cAMP-stimulated test-osterone production and P450c17 mRNA expression. IL-1β completely inhibited cAMP-stimulated testosterone production and P450c17 mRNA expression. PMA is a known activator of protein kinase C (PKC) and has been reported to inhibit Leydig cell steroidogenesis. Leydig cells express type V1 vasopressin receptors, which are coupled to PKC activation. The mechanism of IL-1 action in Leydig cells is not understood, but activation of the PKC pathway has been suggested for IL-1 action in other systems. Therefore, the effects of PMA on cAMP-stimulated steroidogenesis were compared to AVP and IL-1. Similar to the effects of AVP, PMA inhibited cAMP-stimulated testosterone production and P450c17 mRNA expression. To assess the possible involvement of PKC in AVP and IL-1 action in Leydig cells, the PKC inhibitor Calphostin C was tested. cAMP-stimulated testosterone production and P450c17 mRNA expression were significantly inhibited by 10 nM AVP (p < 0.05), and this inhibition was reversed by treatment with Calphostin C. Analogous experiments were performed to assess the role of PKC in IL-1 action. In contrast to the results for AVP, Calphostin C did not reverse the inhibitory effects of IL-1 on cAMP-stimulated P450c17 mRNA expression. To assess further PKC activation, myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation was analyzed. Only AVP and PMA, but not IL-1β, caused an increase in MARCKS phosphorylation. These results confirm that AVP and PMA activate PKC and indicate that IL-1 likely does not activate PKC in Leydig cells. The implications of AVP-mediated inhibition of steroidogenesis and potential role of MARCKS phosphorylation are discussed.
Similar content being viewed by others

References
Bosmann, H. B. Hales, K. H., Li, X., Lui, Z., Stocco, D. M., and Hales, D. B. (1996). Endocrinology 137, 4522–4525.
Hales, D. B., Hales, K. H., and Bosmann, H. B. (199). 2nd International Symposium on Molecular Steroidogenesis. Monterret, CA.
Xiong, Y., and Hales, D. B. (1994). Endocr. J. 2, 223–228.
Turnbull, A. V., and River, C. (1996). Endocrinology 137, 455–463.
Cronenwett, J. L., Baver-Neff, B. S., Grekin, R. J., and Sheagren, J. N. (1986) J. Surg. Res. 41, 609–619.
Egan, J. W., Jugus, M., Kinter, L. B., Lee, K., and Smith, E. F. I. (1989). Circ. Shock. 29, 155–166.
Kasting, N. W., Mazurek, M. F., and Martin, J. B., (1985). Am. J. Physiol. 248, E420–424.
Kasting, N. W. (1989). Can. J. Physiol. Pharm. 66, 22–26.
Schaller, M. D., Waeber, B., Nussberger, J., and Brunner, H. R. (1985). Am. J. Physiol. 249, H1086–1092.
Kasson, B. G., Adashi, E. Y., and Hsueh, A. J. W. (1986). Endocr. Rev. 7, 156–168.
Saez, J. M. (1994). Endocr. Rev. 15, 574–626.
Tahri-Joutei, A. and Pointis, G. (1988). Life Sciences 43, 177–185.
Sayeed, M. M. (1996). New Horm. 4, 72–86.
Nielson, J. R., Hansen, H. S., and Jensen, B. (1989). Mol. Cell Endocrinol. 61, 181–188.
Vinggaard, M. A., and Hansen, H. S. (1991). Mol. Cell Endocrinol. 79, 157–165.
Ascoli, M., Pignataro, O. P., and Segaloff, D. L. (1989). J. Biol. Chem. 264, 6674–6681.
Hales, D. B. (1992). Endocrinology 131, 2165–2172.
Hales, D. B., Xiong, Y., and Tur-Kaspa, I. (1992). J. Steroid Biochem. Mol. Biol. 43, 907–914.
Xiong, Y. and Hales, D. B. (1993). Endocrinology 132, 2438–2444.
Li, X., Youngblood, G. L., Payne, A. H., and Hales, D. B. (1995). Endocrinology 136, 3519–3526.
Dix, C. J., Habberfield, A. D., and Cooke, B. A. (1987). Biochem. J. 243, 373–377.
Moger, W. H. (1985). Life Sci. 37: 869–873.
Mukhopadhyay, A. K., Bohnet, H. G., and Leindenberger, F. A. (1984). Biochem. Biophys. Res. Commun. 119, 1062–1067.
Mukhopadhyay, A. K. and Schumacher, M. (1985). FEBS Letts. 187, 56–60.
Papadopoulos, V., Careau, S., and Drosdowsky, M. A. (1985). FEBS Letts. 188, 313–316.
Rebois, R. V., and Patel, J. (1985). J. Biol. Chem. 260, 8026–8031.
Bankers-Fulbright, J. L., Kalli, K. R., and McKean, D. J. (1996). Life Sci. 59: 61–83.
Kikkawa, U. and Nishizuka, Y. (1986). Annu. Rev. Cell Biol. 2, 149–178.
Aderem, A. (1995). Biochem. Soc. Trans. 23, 587–591.
Aderem, A. (1992). Cell 71, 713–716.
Blackshear, P. J. (1993). In: Handbook of Endocrine Research Techniques. de Pablo, F., Scanes, C. G., and Weintraub, B. D., (eds.). Academic, San Diego, CA, pp. 339–355.
Lobaugh, L. A. and Blackshear, P. J. (1990). J. Biol. Chem. 265, 18,393–18,399.
Liu, J. P., Engler, D., Funder, J. W., and Robinson, P. J. (1994) Mol. Cell Endocrinol. 105, 217–226.
Vinggaard, A. M. and Hansen, H. S. (1993). J. Endocrinol. 136, 119–126.
Nishizuka, Y. (1988). Nature 334, 661–665.
Dinarello, C. A. (1996). Blood 87, 2095–2147.
Stewart, R. J. and Marsden, P. A. (1995). Am. J. Kidney Dis. 25, 954–966.
Riches, D. W., Chan, E. D., and Winston, B. W. (1996). Immunobiology 195, 477–490.
Naismith, J. H. and Sprang, S. R. (1996). J. Inflamm. 47: 1–7.
Grell, M. (1995). J. Inflamm. 47, 8–17.
Aderem, A. (1992). Trends Biochem. Sci. 17, 438–443.
Hall, P. F. and Almahbobi, G. (1992). J. Steroid Biochem. Mol. Biol. 43, 769–777.
Rivier, C. and Rivest, S. (1991) Biol. Reprod. 45, 523–532.
Ivell, R., Hunt, N., Hardy, M., Nicholson, H., and Pickering, B. (1992). Mol. Cell. Endocrinol. 89, 54–61.
Foo, N.-C., Carter, D., Murphy, D., and Ivell, R. (1991). Endocrinology 128, 2118–2128.
Fillion, C., Tahri-Joutei, A., Hugues, J. N., Allevard, A. M., Taib, N., and Pointis, G. (1994). Mol. Cell. Endocrinol. 99, 25–30.
Tahri-Joutei, A., Fillion, C., Bedin, M., Hugues, J.-N., and Pointis, G. (1991). Mol. Cell Endocrinol. 79, R21–24.
Tahri-Joutei, A. and Pointis, G. (1989). FEBS Lett. 254, 189–193.
Kasson, B. G. and Tuchel, T. M. (1989) Endocrinology 124, 2777–2784.
Sharpe, R. M. and Cooper, I. (1987). J. Endocrinol. 113, 89–96.
Bardin, C. W., Chen, C.-L. C., Morris, P. L., Gerendai, I., Boitani, C., Liotta, A. S., et al. (1987). Rec. Prog. Horm. Res. 43, 1–28.
Fabri, A., Knox, G., Buczko, E., and Dufau, M. L. (1988). Endocrinology 122, 749–755.
Turnbull, A. and Rivier, C. (1995). Neuroimmunomodulation 2, 224–235.
Pitas, R. E., Innerarity, T., Weinstein, J. N., and Mahley, R. W. (1981). Arteriosclerosis 1, 177–185.
Laemmli, U. K. (1970). Nature 227, 680–685.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hales, D.B., Greene, R. Arginine vasopressin inhibition of cytochrome P450c17 and testosterone production in mouse leydig cells. Endocr 8, 19–28 (1998). https://doi.org/10.1385/ENDO:8:1:19
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ENDO:8:1:19