Abstract
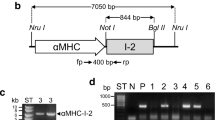
The previously described rabbit 2.3-kilobase smooth muscle myosin haevy-chain (SMHCwt) promoter targets gene expression in transgenic animals to vascular smooth muscle cells (SMCs), including coronary arteries. Therefore, SMHCwt is thought to provide a promising tool for human gene therapy. In the present study, we examined tissue specificity and expression levels of wild-type and mutated SMHC promoters within the system of high-capacity adenoviral (hcAd) vectors. SMHCwt and a series of SMHC promoter deletion mutants, a triple promoter as well as a cytomegalovirus-SMHC hybrid promoter driving the enhanced green fluorescence protein (EGFP) reporter gene were transiently transfected into aortic SMCs. Fluorescence intensity was measured by flow cytometric analysis. Consecutively, hcAd vectors were constructed with the SMHCwt and the mutant promoter with the highest fluorescence activity. Levels of EGFP expression were determined after transduction of SMCs derived from human coronary arteries. For analysis of tissue specificity, embryonic stem (ES) cell-derived SMCs (ESdSMHCs) and cardiomyocytes, (ESdCMs) were used. In comparison with SMHCwt, only the SMHCdel94 mutant lacking a 94-bp GC-rich element revealed a 1.5-fold increased fluorescence activity. Transduction of primary SMCs of human coronary arteries with hcAd vectors confirmed an increased EGFP expression driven by the SMHCdel94 promoter. In ES-cell-derived embryoid bodies, SMHCwt was exclusively active in transduced ESdSMCs. In contrast, expression of SMHCdel94 was also found in ESdCMs and other nontarget cells of the embryoid body. The tissue-specific rabbit SMHCwt promoter seems to be suitable for adenoviral gene transfer in SMCs of human coronary arteries and deletion of a 94-bp negative cis-acting GC-rich element results in loss of specificity.
Similar content being viewed by others
References
Yla-Herttuala, S., and Alitalo, K. (2003) Gene transfer as a tool to induce therapeutic vascular growth. Nat. Med. 9, 694–701.
Shenk, T.E. (2001) Adenoviridae: the viruses and their replication, in Fields Virology, Vol. 1 (Knipe, D. M. and Howley, P. M., eds.), Lippincott-Raven, Philadelphia, pp. 2265–2300.
Flugelman, M. Y., Jaklitsch, M. T., Newman, K. D., Casscells, W., Bratthauer, G. L., and Dichek, D. A. (1992) Low level in vivo gene transfer into the arterial wall through a perforated balloon catheter. Circulation 85, 1110–1117.
Chang, M. W., Barr, E., Lu, M. M., Barton, K., and Leiden, J. M. (1995) Adenovirus-mediated over-expression of the cyclin/cyclin dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J. Clin. Investig. 96, 2260–2268.
Chen, D., Krasinski, K., Sylvester, A., Chen, J., Nisen, P. D., and Andres, V. (1997) Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27 (KIP1), an inhibitor of neointima formation in the rat carotid artery. J. Clin. Investig. 99, 2334–2341.
Janssens, S., Flaherty, D., Nong, Z., et al. (1998) Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation 97, 1274–1281.
Simari, R. D., San, H., Rekhter, M., et al. (1996) Regulation of cellular proliferation and intimal formation following balloon injury in atherosclerotic rabbit arteries. J. Clin. Investig. 98, 225–235.
Guzman, R. J., Hirschowitz, E., Brody, S. L., Crystal, R. G., Epstein, S. E., and Finkel, T. (1995) In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 91, 10,732–10,736.
Zaiss, A. K., Liu, Q., Bowen, G. P., Wong, N. C., Bartlett, J. S., and Muruve, D. A. (2002) Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors, J. Virol. 76, 4580–4590.
Schulick, A. H., Vassalli, G., Dunn, P. F., et al. (1997) Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries: potential of immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99, 202–219.
Newman, K. D., Dunn, P. F., Owens, J. W., et al. (1995) Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation and neointimal hyperplasia. J. Clin. Investig. 96 2955–2965.
Baek, S., and March, K. L. (1998) Gene therapy for restenosis: getting nearer the heart of the matter. Circ. Res. 82, 295–305.
Chuah, M. K., Schiedner, G., Thorrez, L., et al. (2003) Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high capacity adenoviral vectors. Blood 101, 1734–1743.
Jiang, Z., Schiedner, G., van Rooijen, N., Liu, C. C., Kochanek, S., and Clemens, P. R. (2004) Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol. Ther. 10, 688–696.
Schiedner, G., Morral, N., Parks, R. J., et al. (1998) Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18, 180–183.
Pastore, L., Morral, N., Zhou, H., et al. (1999) Use of a liverspecific promoter reduces immune response to the transgene in adenoviral vectors. Hum. Gene Ther. 10, 1773–1781.
Chen, H. H., Mack, L. M., Kelly, R., Ontell, M., Kochanek, S., and Clemens, P. R. (1997) Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl. Acad. Sci. USA 94, 1645–1650.
Rothmann, T., Katus, H. A., Hartong, R., Perricaudet, M., and Franz, W. M. (1996) Heart muscle-specific gene expression using replication defective recombinant adenovirus. Gene Ther. 3, 919–926.
Franz, W. M., Mueller, O. J., Fleischmann, M., et al. (1999) The 2.3 kb smooth muscle myosin heavy chain promoter directs gene expression into the vascular system of transgenic mice and rabbits. Cardiovasc. Res. 43, 1040–1048.
Wang, J., Niu, W., Nikiforov, Y., et al. (1997) Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J. Clin. Investig. 100, 1425–1439.
Li, L., Miano, J. M., Mercer, B., and Olson, E. N. (1996) Expression of the SM22a promotor in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J. Cell Biol. 132, 849–859.
Herring, P. B., and Smith, A. F. (1996) Telokin expression is mediated by a smooth muscle cell-specific promotor. Am. J. Physiol. 270, C1656-C1665.
Madsen, C. S., Hershey, J. C., Hautmann M. B., White, S. L. and Owens, G. K. (1997) Expression of the smooth muscle myosin heavy chain gene is regulated by a negativeacting GC-rich element located between two positive-acting serum response factor-binding elements. J. Biol. Chem. 272, 6332–6340.
Zilberman, A., Dave, V., Miano, J., Olson, E. N., and Periasamy, M. (1998) Evolutionarily conserved promoter region containing CArG*-like elements is crucial for smooth muscle myosin heavy chain gene expression. Circ. Res. 82, 566–575.
Ventura, C., Maioli, M., Asara, Y., et al. (2004) Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J. Biol. Chem. 279, 23,574–23,579.
Wobus, A. M., Kaomei, G., Shan, J., et al. (1997) Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 29, 1525–1539.
Drab, M., Haller, H., Bychkov, R., et al. (1997) From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. 11, 905–915.
Schreiber, K. L., Calderone, A., and Rind, H. (2000) Distant upstream regulatory domains direct high levels of betamyosin heavy chain gene expression in differentiated embryonic stem cells. J. Mol. Cell. Cardiol. 32, 585–598.
Muller, M., Fleischmann, B. K., Selbert, S., et al. (2000) Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J. 14, 2540–2548.
Brevetti, L. S., Chang, D. S., Tang, G. L., Sarkar, R., and Messina, L. M. (2003) Overexpression of endothelial nitric oxide synthase increases skeletal muscle blood flow and oxygenation in severe rat hind limb ischemia. J. Vasc. Surg. 38, 820–826.
Kondoh, K., Koyama, H., Miyata, T., Takato, T., Hamada, H., and Shigematsu, H. (2004) Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc. Res. 16, 132–142.
Luo, Z., Akita, G. Y., Date, T., et al. (2005) Adenovirusmediated expression of beta-adrenergic receptor kinase C-terminus reduces intimal hyperplasia and luminal stenosis of arteriovenous polytetrafluoroethylene grafts in pigs. Circulation 111, 1679–1684.
Kim, S., Lin, H., Barr, E., Chu, L., Leiden, J. M., and Parmacek, M. S. (1997) Transcriptional targeting of replication-defective adenovirus transgen expression to smooth muscle cells in vivo. J. Clin. Investig. 100, 1006–1014.
Vigne, E., Mahfouz, I., Dedieu, J. F., Brie, A., Perricaudet, M., and Yeh, P. (1999) RGD inclusion in the hexon monomer provides adenovirus type 5 based vectors with a fiber knob-independent pathway for infection. J. Virol. 73, 5156–5161.
Wickham, T. J., Tzeng, E., Shears, L., et al. (1997) Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 71, 8221–8229.
White, S. L., and Low, R. B. (1996) Identification of promoter elements involved in cell specific regulation of rat smooth muscle myosin heavy chain gene transcription. J. Biol. Chem. 271, 15,008–15,017.
Sartorelli, V., Webster, K. A., and Kedes, L. (1990) Musclespecific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 4, 1811–1822.
Hagstrom, J. N., Couto, L. B., Scallan, C., et al. (2000) Improved muscle-derived expression of human coagulation factor IX from a skeletal actin/CMV hybrid enhancer/promoter. Blood 95, 2536–2542.
Jin, Y., Pasumarthi, K. B., Bock, M. E., Chen, Y., Kardami, E., and Cattini, P. (1995) Effect of “enhancer” sequences on ventricular myosin light chain-2 promoter activity in heart muscle and nonmuscle cells. Biochem. Biophys. Res. Commun. 210, 260–266.
Liu, R., Baillie, J., Sissons, J. G., and Sinclair, J. H. (1994) The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in nonpermissive cells. Nucleic Acids Res. 22, 2453–2459.
Kothari, S., Baillie, J., Sissons, J. G., and Sinclair, J. H. (1991) The 21bp repeat element of the human cytomegalovirus major immediate early enhancer is a negative regulator of gene expression in undifferentiated cells. Nucleic Acids Res. 19, 1767–1771.
Edwards, A., Voss, H., Rice, P., et al. (1990) Automated DNA sequencing of the human HPRT locus. Genomics 6, 593–560.
Consalez, G. G., Stayton, C. L., Freimer, N. B., et al. (1992) Isolation and characterization of a highly polymorphic human locus (DXS455) in proximal Xq28. Genomics 12, 710–714.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to the study.
Rights and permissions
About this article
Cite this article
Deindl, E., Middeler, G., Müller, O.J. et al. Identification of a 94-bp GC-rich element in the smooth muscle myosin heavy-chain promoter controlling vascular smooth muscle cell-specific gene expression. Cell Biochem Biophys 45, 279–288 (2006). https://doi.org/10.1385/CBB:45:3:279
Issue Date:
DOI: https://doi.org/10.1385/CBB:45:3:279