Abstract
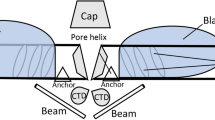
Activation and inactivation of ion channels involve volume changes from conformal rearrangements of channel proteins. These volume changes are highly susceptible to changes in ambient pressure. Depending on the pressure level, channel function may be irreversibly altered by pressure. The corresponding structural changes persist through the post-decompression phase. High-pressure applications are a useful tool to evaluate the pressure dependence as well as pressure limits for reversibility of such alterations. Mammalian cells are only able to tolerate much lower pressures than microorganisms. Although some limits for pressure tolerance in mammalian cells have been evaluated, the mechanisms of pressure-induced alteration of membrane physiology, in particular of channel function, are unknown. To address this question, we recorded fast inward sodium (INa) and slowly activating L-type calcium (ICa) currents in single mammalian muscle fibers in the post-decompression phase after a prolonged 3-h, high-pressure treatment of up to 20 MPa. INa and ICa peak amplitudes were markedly reduced after pressure treatment at 20 MPa. This was not from a general breakdown of membrane integrity as judged from in situ high-pressure fluorescence microscopy. Membrane integrity was preserved even for pressures as high as 35 MPa at least for pressure applications of shorter durations. Therefore, the underlying mechanisms for the observed amplitude reductions have to be determined from the activation (time-to-peak [TTP]) and inactivation (τdec) kinetics of INa and ICa. No major changes in INa kinetics, but marked increases, both in TTP and τdec for ICa, were detected after 20 MPa. The apparent molecular volume changes (activation volumes) ΔV ‡ for the pressure-dependent irreversible alteration of channel gating approached zero for Na+ channels. For Ca2+ channels, ΔV‡ was very large, with approx 2.5-fold greater values for channel activation than inactivation (approx 210 Å3). We conclude, that in skeletal muscle, high pressure differentially and irreversibly affects the gating properties and the density of functional Na+ and Ca2+ channels. Based on these results, a model of high pressure-induced alterations to the channel conformation is proposed.
Similar content being viewed by others
References
Macdonald, A. G. (2002) Experiments on ion channels at high pressure. Biochim. Biophys. Acta 1595, 387–389.
Hall, A. C., Pickles, D. M., and Macdonald, A. G. (1993) Aspects of eukaryotic cells, in Effects of High Pressure on Biological Systems (Macdonald, A. G., ed.). Springer Verlag Heidelberg, New York, 1993, pp. 30–85.
Ornhagen, H. C., and Sigurdsson, S. B. (1981) Effects of high hydrostatic pressure on rat atria muscle. Undersea Biomed. Res. 8, 113–120.
Goldinger, J. M., Kang, B. S., Choo, Y. E., Paganelli, C. V., and Hong, S. K. (1980) Effect of hydrostatic pressure on ion trasport and metabolism in human erythrocytes. J. Appl. Physiol. Respir. Environ. Exercise Physiol. 49, 224–231.
Crenshaw, H. C., Allen, J. A., Skeen, V., Harris, A., and Salmon, E. D. (1996) Hydrostatic pressure, has different effects on the assembly of tubulin, actin, myosin II, vinculin, talin, vimentin and cytokeratin in mammalian tissue cells. Exp. Cell Res. 227, 285–297.
Macdonald, A. G. (1997) Effect of high hydrostatic pressure on the BK channel in bovine chromaffin cells. Biophys. J. 73, 1866–1873.
Kress, K. R., Friedrich, O., Ludwig, H., and Fink, R. H. A. (2001) Reversibility of high pressure effects on the contractility of skeletal muscle. J. Mus. Res. Cell Mot. 22, 379–389.
Friedrich, O., Kress, K. R., Ludwig, H., and Fink, R. H. A. (2002) Membrane ion conductances of mammalian skeletal muscle in the post-decompression state after high pressure treatment. J. Membr. Biol. 188, 11–22.
Kaarniranta, K., Elo, M. A., Sironen, R. K., Karjalainen, H. M., Helminen, H. J., and Lammi, M. J. (2003) Stress response of mammalian cells to high hydrostatic pressure. Biorheology 40, 87–92.
Conti, F., Fiovaranti, R., Segal, J. R., and Stühmer, W. (1982) Pressure dependence of the sodium currents of squid giant axon. J. Membr. Biol. 69, 23–34.
Conti, F, Fiovaranti, R., Segal, J. R., and Stühmer, W. (1982) Pressure dependence of the potassium currents of squid giant axon. J. Membr. Biol. 69, 35–40.
Iwasaki, T. and Yamamoto, K. (2002) Effect of high hydrostatic pressure on chicken myosin subfragment-1. Int. J. Biol. Macromol. 30, 227–232.
Smeller, L. (2002) Pressure-temperature phase diagrams of biomolecules. Biochim. Biophys. Acta 1595, 11–29.
De Felice, F. G., Soares, V. C., and Ferreira, S. T. (1999) Subunit dissociation and inactivation of pyruvate kinase by hydrostatic pressure. Eur. J. Biochem. 266, 163–169.
Gross, M. and Jaenicke, R. (1994) Proteins under pressure. The influence of high hydrostatic pressure on structure, function, and assembly of proteins and protein complexes. Eur. J. Biochem. 221, 617–630.
Sharma, A., Scott, J. H., Cody, G. D., Fogel, M. L., Hazen, R. M., Hemley, R. J., and Huntress, W. T. (2002) Microbial activity at gigapascal pressures. Science 295, 1514–1516.
Heinemann, S. H., Conti, F., Stühmer, W., and Neher, E. (1987) Effects of high pressure on membrane processes: sodium channels, calcium channels, and exocytosis. J. Gen. Physiol. 90, 765–778.
Kontis, K. J. and Goldin, A. L. (1997) Sodium channel inactivation is altered by substitution of voltage sensor positive charges. J. Gen. Physiol. 110, 403–413.
Chen, L. Q., Santarelli, V., Horn, R., and Kallen, R. G. (1996) A unique role of the S4 segment of domain 4 in the inactivation of sodium channels. J. Gen. Physiol. 108, 549–556.
Groome, J. R., Fujimoto, E., George, A. L., and Ruben, P. C. (1999) Differential effects of homologous S4 mutations in human skeletal muscle sodium channels on deactivation gating from open and inactivated states. J. Physiol. 516, 687–698.
Stühmer, W., Conti, F., Suzuki, H., et al. (1989) Structural parts involved in activation and inactivation of the sodium channel. Nature 339, 597–603.
Bezanilla, F. (2002) Perspective: voltage-sensor movements. J. Gen. Physiol. 120, 465–473.
Chanda, B. and Bezanilla, F. (2002) Tracking voltage-dependent conformational changes in skeletal muscle sodium channels during activation. J. Gen. Physiol. 120, 629–645.
Meyer, R. and Heinemann, S. H. (1997) Temperature and pressure dependence of Shaker K+ channel N- and C-type inactivation. Eur. Biophys. J. 26, 433–445.
Weber, G. and Drickamer, H. G. (1983) The effect of high pressure upon proteins and other biomolecules. Q. Rev. Biophys. 16, 89–112.
Kendig, J. J. (1984) Ionic currents in vertebrate myelinated nerve at hyperbaric pressure. Amer. J. Physiol. 246, C84-C90.
Taulier, N. and Chalikian, T.V. (2002) Compressibility of protein transients. Biochim. Biophys. Acta 1595, 48–70.
Harper, A. A. Macdonald, A. G., and Wann, K. T. (1981) The action of high hydrostatic pressure on the membrane currents of Helix neurones. J. Physiol. 311, 325–339.
Grossman, Y. and Kendig, J. J. (1984) Pressure and temperature: time-dependent modulation of membrane properties in a bifurcating axon. J. Neurophysiol. 52, 692–708.
Jurkat-Rott, K. and Lehmann-Horn, F. (2001) Human muscle voltage-gated ion channels and hereditary disease. Cur. Opinion Pharmacol. 1, 280–287.
Hall, A. C., Ellroy, J. C., and Klein, R. A. (1982) Pressure and temperature effects on human red cell cation transport. J. Membr. Biol. 68, 47–56.
Friedrich, O., Ehmer, Th., and Fink, R. H. A. (1999) Calcium currents during contraction and shortening in enzymatically isolated murine skeletal muscle fibers. J. Physiol. 517, 757–770.
Hu, L. Y., Tian, S. M., Ye, Q. Z., and Ruan, K. C. (2000) Comparison of the catalytic domains of collagenase-1 and stromelysin-1. Sheng Wu Hua xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 32, 409–412.
Friedrich, O., Ehmer, Th., Uttenweiler, D., Vogel, M., Barry, P. H., and Fink, R. H. A. (2001) Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys. J. 80, 2046–2055.
Desaphy, J. F., Pierno, S., Leoty, C., George, A. L. Jr., De Luca, A., and Conte Camerino, D. (2001) Skeletal muscle disuse induces fibre type-dependent enhancement of Na+ channel expression. Brain 124, 1100–1113.
Macdonald, A. G. (2001) Effects of high pressure on cellular processes, in Cell Physiology Source Book: A Molecular Approach (Sperelakis, N., ed.). 3rd ed. Academic Press, San Diego, CA, pp. 1003–1023.
Hartmann, M., Kreuss, M., and Sommer, K. (2004) High pressure microscopy—a powerful tool for monitoring cells and macromolecules under high hydrostatic pressure. Cell. Mol. Biol. 50, 479–484.
Hartmann, M., Pfeifer, F., Dornheim, G., and Sommer, K. (2003) HPDS—Hochdruckzelle zur Beobachtung mikroskopischer Phänomene unter Hochdruck. Chemie Ingenieur Technik 75, 1763–1767.
Macklis, J. D. and Madison, R. D. (1990) Progressive incorporation of propidium iodide in cultured mouse neurons correlates well with declining electrophysiological status: a fluorescence scale of membrane integrity. J. Neurosci. Methods 31, 43–46.
Scoltock, A. B., Bortner, C. D., St.-J. Bird, G., Putney, J. W. Jr., and Cidlowski, J. A. (2000) A selective requirement for elevanted calcium in DNA degradation, but not early events in anti-FAS-induced apoptosis. J. Biol. Chem. 275, 30586–30596.
Crenshaw, H. C. and Salmon, E. D. (1996) Hydrostatic pressure to 400 atm does not induce changes in the cytosolic concentration of Ca2+ in mouse fibroblasts: measurements using fura-2 fluorescence. Exp. Cell Res. 227, 285–297.
Kirsch, W. G., Uttenweiler, D., and Fink, R. H. A. (2001) Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J. Physiol. 537, 379–389.
Mannuzzu, L. M. and Isacoff, E. Y. (2000) Independence and cooperativity in rearrangements of a potassium channel voltage sensor revealed by single subunit fluorescence. J. Gen. Physiol. 115, 257–268.
Friedrich, O., Both, M., Gillis, J. M., Chamberlain, J. S., and Fink, R. H. A. (2004) Mini-dystrophin restores L-type calcium currents in skeletal muscle of transgenic mdx mice. J. Physiol. 555, 251–265.
Gee, S. H., Madhavan, R., Levinson, S. R., Caldwell, J. H., Sealock, R., and Froehner, S. C. (1998) Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci. 18, 128–137.
Gomez, A. M., Kerfant, B. G., and Vassort, G. (2000) Microtubule disruption modulates Ca2+ signalling in rat cardiac myocytes. Circ. Res. 86, 30–36.
Verjovski-Almeida, S., Kurtenbach, E., Amorim, A. F., and Weber, G. (1986) Pressure-induced dissociation of solubilized sarcoplasmic reticulum ATPase. J. Biol. Chem. 261, 9872–9878.
Coelho-Sampaio, T., Ferriera, S. T., Benaim, G., and Vieyra, A. (1991) Dissociation of purified erythrocyte Ca2+ ATPase by hydrostatic pressure. J. Biol. Chem. 266, 22266–22272.
Pin, S., Royer, C. A., Gratton, E., Alpert, B., and Weber, G. (1990) Subunit interactions in haemoglobin probed by fluorescence and high pressure techniques. Biochemistry 29, 9194–9202.
Smeller, L., Rubens, P., and Heremans, K. (1999) Pressure effect on the temperature induced unfolding and tendency to aggregate of myoglobin. Biochemistry 38, 3816–3820.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedrich, O., Kress, K.R., Hartmann, M. et al. Prolonged high-pressure treatments in mammalian skeletal muscle result in loss of functional sodium channels and altered calcium channel kinetics. Cell Biochem Biophys 45, 71–83 (2006). https://doi.org/10.1385/CBB:45:1:71
Issue Date:
DOI: https://doi.org/10.1385/CBB:45:1:71