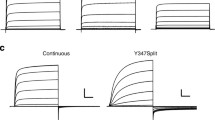
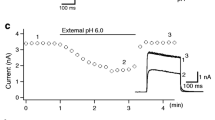
Abstract
Extracellular acidification and reduction of extracellular K+ are known to decrease the currents of some voltage-gated potassium channels. Although the macroscopic conductance of WT hKv1.5 channels is not very sensitive to [K+]o at pH 7.4, it is very sensitive to [K+]o at pH 6.4, and in the mutant, H463G, the removal of K+ o virtually eliminates the current at pH 7.4. We investigated the mechanism of current regulation by K+ o in the Kv1.5 H463G mutant channel at pH 7.4 and the wild-type channel at pH 6.4 by taking advantage of Na+ permeation through inactivated channels. Although the H463G currents were abolished in zero [K+]o, robust Na+ tail currents through inactivated channels were observed. The appearnnce of H463G Na+ currents with a slow rising phase on repolarization after a very brief depolarization (2 ms) suggests that channels could activate directly from closed-inactivated states. In wild-type channels, when intracellular K+ was replaced by NMG+ and the inward Na+ current was recorded, addition of 1 mM K+ prevented inactivation, but changing pH from 7.4 to 6.4 reversed this action. The data support the idea that C-type inactivation mediated at R487 in Kv1.5 channels is influenced by H463 in the outer pore. We conclude that both acidification and reduction of [K+]o inhibit Kv1.5 channels through a common mechananism (i.e., by increasing channel inactivation, which occurs in the resting state or develops very rapidly after activation).
Similar content being viewed by others
References
Eisner, D. A., Nichols, C. G., O'Neill, S. C., Smith, G. L., and Valdeolmillos, M. (1989) The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J. Physiol. (Camb.) 411, 393–418.
Orchard, C. H. and Kentish, J. C. (1990) Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. Cell Physiol. 258, C967-C981.
Yan, G-X. and Kléber, A. G. (1992) Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ. Res. 71, 460–470.
Steidl, J. V. and Yool, A. J. (1999) Diferential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Molec. Pharmacol. 55, 812–820.
Kehl, S. J., Eduljee, C., Kwan, D. C. H., Zhang, S., and Fedida, D. (2002) Molecular determinants of the inhibition of human Kv1.5 potassium currents by external protons and Zn2+. J. Physiol. (Lond.) 541, 9–24.
Jäger, H. and Grissmer, S. (2001) Regulation of a mammalian Shaker-related potassium channel, hKv1.5, by extracellular potassium and pH. FEBS Lett. 488, 45–50.
Perez-Cornejo, Stampe, P., and Begenisich, T. (1998) Proton probing of the charybdotoxin binding site of Shaker K+ channels. J. Gen. Physiol. 111, 441–450.
Starkus, J. G., Varga, Z., Schonherr, R., and Heinemann, S. H. (2003) Mechanisms of the inhiition of Shaker potassium channels by protons. Pflugers Arch. 447, 44–54.
Claydon, T. W., Boyett, M. R., Sivaprasadarao, A., Ishii, K., Owen, J. M., O'Beirne, H. A., Leach, R., Komukai, K., and Orchard, C. H. (2000) Inhibition of the K+ channel Kv1.4 by acidosis: protonation of an extracellular histidine slows the recovery from N-type inactivation. J. Physiol. (Camb.) 526, 253–264.
Ishii, K., Nunoki, K., Yamagishi, T., Okada, H., and Taira, N. (2001) Differential sensitivity of Kv1.4, Kv1.2, and their tandem channel to acidic pH: Involvement of a histidine residue in high sensitivity to acidic pH. J. Pharmacol. Exp. Ther. 296, 405–411.
Pardo, L. A., Heinemann, S. H., Terlau, H., Ludewig, U., Lorra, C., Pongs, O., and Stühmer, W. (1992) Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. U S A 89, 2466–2470.
Jäger, H., Rauer, H., Nguyen, A. N.., Aivar, J., Chandy, K. G., and Grissmer, S. (1998) Regulation of mammalian Shaker-related K+ channels: evidence for non-conducting closed and non-conducting inactivated states. J. Physiol. (Camb.) 506, 291–301.
Lopez-Barneo, J., Hoshi, T., Heinemann, S. H., and Aldrich, R. W. (1993) Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Recept. Channels 1, 61–71.
Starkus, J. G., Kuschel, L., Rayner, M. D., and Heinemann, S. H. (1997) Ion conduction through C-type inactivated Shaker channels. J. Gen. Physiol. 110, 539–550.
Wang, Z. and Fedida, D. (2001) Gating charge immobilization caused by the transition between inactivated states in the Kv1.5 channel. Biophys. J. 81, 2614–2627.
Wang, Z. R., Hesketh, J. C., and Fedida, D. (2000) A high-Na+ conduction state during recovery from inactivation in the K+ channel Kv1.5. Biophys. J. 79, 2416–2433.
Zhang, S., Kehl, S. J., and Fedida, D. (2003) Modulation of human ether-a-go-go-related K+ (hERG) channel inactivation by Cs+ and K+. J. Physiol. (Camb.) 548, 691–702.
Zhang, S., Kurata, H. T., Kehl, S. J., and Fedida, D. (2003) Rapid induction of P/C-type inactivation is the mechanism for acid-induced K+ current inhibition. J. Gen. Physiol. 121, 215–225.
Chen, F. S. P., Steele, D., and Fedida, D. (1997) Allosteric effects of permeating cations on gating currents during K+ channel deactivation. J. Gen. Physiol. 110, 87–100.
Yang, Y. S., Yan, Y. Y., and Sigworth, F. J. (1997) How does the W434F mutation block current in Shaker potassium channels. J. Gen. Physiol. 109, 779–789.
Zhang, S. T., Kehl, S. J., and Fedida, D. (2001) Modulation of Kv1.5 potassium channel gating by extracellular zinc. Biophys. J., 81, 125–136.
Doyle, D. A., Cabral, J. M., Pfuetzner, R. A., Kuo, A. L., Gulbis, J. M., Cohen, S. L., Chait, B. T., and MacKinnon, R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selective. Science 280, 69–77.
Fedida, D., Wible, B., Wang, Z., Fermini, B., Faust, F., Nattel, S., and Brown, A. M. (1993) Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 73, 210–216.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Eduljee, C., Kwan, D.C.H. et al. Constitutive inactivation of the hKv1.5 mutant channel, H463G, in K+-free solutions at physiological pH. Cell Biochem Biophys 43, 221–230 (2005). https://doi.org/10.1385/CBB:43:2:221
Issue Date:
DOI: https://doi.org/10.1385/CBB:43:2:221