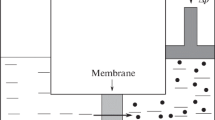
Abstract
In 1903, George Hulett explained how solute alters water in an aqueous solution to lower the vapor pressure of its water. Hulett also explained how the same altered water causes osmosis and osmotic pressure when the solution is separated from liquid water by a membrane permeable to the water only. Hulett recognized that the solute molecules diffuse toward all boundaries of the solution containing the solute. Solute diffusion is stopped at all boundaries, at an open-unopposed surface of the solution, at a semipermeable membrane, at a container wall, or at the boundary of a solid or gaseous inclusion surrounded by solution but not dissolved in it. At each boundary of the solution, the solute molecules are reflected, they change momentum, and the change of momentum of all reflected molecules is a pressure, a solute pressure (i.e., a force on a unit area of reflecting boundary). When a boundary of the solution is open and unopposed, the solute pressure alters the internal tension in the force bonding the water in its liquid phase, namely, the hydrogen bond. All altered properties of the water in the solution are explained by the altered internal tension of the water in the solution. We acclaim Hulett's explanation of osmosis, osmotic pressure, and lowering of the vapor pressure of water in an aqueous solution. His explanation is self-evident. It is the necessary, sufficient, and inescapable explanation of all altered properties of the water in the solution relative to the same property of pure liquid water at the same externally applied pressure and the same temperature. We extend Hulett's explanation of osmosis to included the osmotic effects of solute diffusing through solvent and dragging on the solvent through which it diffuses. Therein lies the explanations of (1) the extravasation from and return of interstitial fluid to capillaries, (2) the return of luminal fluid in the proximal and distal convoluted tubules of a kidney nephron to their peritubular capillaries, (3) the return of interstitial fluid to the vasa recta, (4) return of aqueous humor to the episcleral veins, and (5) flow of phloem from source to sink in higher plants and many more examples of fluid transport and fluid exchange in animal and plant physiology. When a membrane is permeable to water only and when it separates differing aqueous solutions, the flow of water is from the solution with the lower osmotic pressure to the solution with the higher osmotic pressure. On the contrary, when no diffusion barrier separates differing parts of an aqueous solution, fluid may flow from the part with the higher osmotic pressure to the part with the lower osmotic pressure because the solute molecules diffuse toward their lower concentration and they drag on the water through which they diffuse. This latter osmotic effect (diffusing solute dragging on solvent or counterosmosis) between differing parts of a solution has long been neglected and ignored when explaining fluid fluxes in plant and animal physiology. For two solutions separated by a semipermeable membrane, osmosis is the flow of its solvent from the solution with the lower solute concentration into the solution with the higher solute concentration. For two contiguous solutions not separated by a semipermeable membrane, counterosmosis is the flow of solution with the higher solute concentration toward the solution with the lower solute concentration. Corrective treatment of medical disorders attributable to faulty distribution of body fluids (e.g., glaucoma, pulmonary edema, systemic edema) are possible with these new insights regarding fluid transport and exchange provided in this review.
Similar content being viewed by others
References
Hulett, G. (1903), Beziehung zwischen negativem Druck und osmotischem Druck. Z. Phys. Chem. 42, 353–368.
Lewis, G. N. (1908) The osmotic pressure of concentrated solutions, and the laws of the perfect solution. J. Am. Chem. Soc. 30, 668–683.
Laidler, K. J. (1993), The World of Physical Chemistry, Oxford University Press, NY.
Hammel, H. T. and Scholander, P. F. (1976) Osmosis and Solvent Tension, Springer-Verlag, Berlin.
Dutrochet, R. J. H. (1828) Nouvelles recherches sur l'endosmose et l'exoosmose. JB Ballière, Paris.
Traube, M. (1867) Experimente zur theorie der zellenbildung und endosome. Arch. Anat. Physiol. Wiss. Med. 87–165
Krogh, A. (1959), The Anatomy and Physiology of Capillaries. Hafner Publishing Company, New York.
Pfeffer, W. (1877), Osmotische Untersuchungen, Studien zur Zellenmechanik, Leipzig. Translation into English in Harper's Scientific Memoirs (Ames, J. S. and Jones, H. C. eds.), New York, 1899.
van't Hoff, J. H. (1886) Une propriété général de la matiére diluée. Svenska Vet. Akad. Handl. 21, 17, 43.
van't Hoff, J. H. (1886) Lois de l'équilibre chemique dans l'état dilus gazeux ou dissous. Svenska Vet. Akad. Handl. 21, 17, 217.
van't Hoff, J. H. (1887) Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen. Z. Physik. Chem. 1, 481–508.
Hammel, H. T. and Schlegel, W. W. (2003) Explaining osmosis: by altered water concentration or by altered internal water tension. FASEB J. 8, 14.
van't Hoff, J. H. (1892), Zur Theorie der Lösungen. Z. Physik. Chem. 9, 477.
Meyer, L. (1890) Uber das Wessen des osmotischen Druckes. Z. Phys. Chem. 7, 23–27.
Fermi, E. (1936) Thermodynamics, Dover Publications Inc.
Scholander, P. F., Hammel, H. T., Bradstreet, E. D., and Hemmingsen, E. A. (1965) Sap pressure in vascular plants. Science 148, 339–347.
Chinard, F. P. and Enns, T. (1956) Osmotic pressure. Science 124, 473–474.
Scholander, P. F. (1971) State of water in osmotic processes. Microvasc. Res. 3, 215–232.
Mysels, K. (1959). Introduction to Colloid Chemistry. Interscience Publishers, Inc., New York.
Mysels, K. J. (1978) Solvent tension or solvent concentration. J. Chem. Ed. 55, 21–22.
Mysels, K. J. (1997) Vapor pressure lowering, somotic pressure, and the elementary pseudogas model. J. Phys. Chem. B. 101, 1893–1896.
Hammel, H. T. and Scholander, P. F. (1973) Thermal motion and forced migration of colloidal particles generate hydrostatic pressure in solvent. Proc. Nat. Acad. Sci. U.S.A. 70, 124–129.
Hammel, H. T. and Scholander, P. F. (1976) Osmosis and Tensile Solvent. Springer-Verlag, Berlin-New York.
Hammel, H. T. (1976) Colligative properties of a solution: enhanced tension in the solvent gives rise to alteration in solution. Science 192, 748–756.
Hammel, H. T. (1986) Solubility and enhanced altered tension of solute in solution. Phys. Chem. Liq. 15, 185–202.
Hammel, H. T. (1979) Forum on Osmosis. I. Osmosis: diminished solvent activity or altered solvent tension? Am. J. Physiol. 237, R95-R107.
Hammel, H. T. (1994) How solutes alter water in aqueous solutions. J. Phys. Chem. 98, 4196–4204.
Hammel, H. T. (1998) Replacing Lewis' theory of osmosis with Hulett's theory of altered chemical potentials of reacting constituents in solution. Recent Res. Dev. Phys. Chem. 2, 77–111.
Hammel, H. T. (1999) Evolving ideas about osmosis and capillary fluid exchange FASEB J. 13, 213–223.
Hildebrand, J. H. (1979) Forum on osmosis: II. A criticism of “solvent tension” in osmosis. Am. J. Physiol. 237, R110-R113.
Mauro, A. (1997). Forum on Osmosis. III. Comments on Hammel and Schlolander's solvent tension theory and its application to osmotic flow. Am. J. Physiol. 237, R108-R109.
Soodak, H. and Iberall, A (1979) Forum on osmosis IV. More on osmosis and diffusion. Am. J. Physiol. 237, R114-R122.
Andrews, F. C. (1976) Colligative properties of simple solutions: solutes simply dilute the solvent; they do not cause tension in the solvent. Science 194, 567–571.
Lachish, U. L. (1978) Derivation of some basic properties of ideal gases and solutions from processes of elastic collisions. J. Chem. Ed. 55, 369–371.
Katz, M. A. and Bresler, E. H. (1984) Osmosis, in Edema (Staub, N. C. and Taylor, A. E., eds.) Raven Press, New York.
Ben-Sasson, S. A. and Grover, N. B. (2003) Osmosis: a macroscopic phenomenon, a microscopic view. Adv. Physiol. Ed., 27, 15–19.
West, J. B. (1990) Best and Taylor's Physiological Basis of Medical Practice 12th ed. Williams & Wilkins, Baltimore.
Withers, P. C. (1992) Comparative Animal Physiology, Saunders College Publishing HBJ, Philadelphia.
Pauling, L. (1964), College Chemistry: An Introductory Textbook of General Chemistry: Section 17–18; W. H. Freeman & Co., San Francisco.
Schultz, S. G. (1980) Basic Principles of Membrane Transport. Cambridge University Press, New York.
Lewis, G. N. and Randall, M. (1961) Thermodynamics (revised by Pitzer, K. S. and Brewer, L. II, eds.) McGraw-Hill, New York.
Raoult, F. M. (1878) Sur la tension de vapeur et sur le point de cong'elation de solutions salines. Compt. Rend. 87, 167–171.
Raoult, F. M. (1883) Lo de cong'elation des solutions aqueuses des materl'eres organiques. Ann. Chim. Phys. 28, 133–144.
Raoult, F. M. (1882) Loi de cong'elation des solutions benzeniques des substances neutres. Comput. Rend. 95, 187.
Raoult, F. M. (1882) Loi de geng'elation des dissolvents. Compt. Rend. 95, 1030–1033.
Schermer, M. (2002) Smart people believe weird things—rarely does anyone weigh the facts before deciding what to believe. Sci. Am. 287, 35.
Guyton, A. C. and Hall, J. E. (2000) Textbook of Medical Physiology. 10th ed. W. B. Saunders Co., Philadelphia.
Millero, F. G. and Knox, J. H. (1973) Apparent molal volumes of aqueous NaF, Na2SO4, KCl, K2SO4, MgCl2 and MgSO4 solutions at 0°C and 50°C. J. Chem. Eng. Data 18, 407–411.
Ussing, N. S. (1952) Some aspects of the application of tracers in permeability studies. Adv. Enzymol. 13, 21.
Pappenheimer, J. R. (1953) Passage of molecules through capillary walls. Physiol. Rev. 33, 389–423.
Mauro, A. (1957) Nature of solvent transfer in osmosis. Science 126, 252–253.
Meschia, G. and Setnikar, I. (1959) Experimental study of osmosis through a collodion membrane. J. Gen. Physiol. 42, 429–444.
Dobson, H. J. E. (1925) The partial pressures of aqueous ethyl alcohol. J. Chem. Soc. (London) 127, 2866–2873.
Dixon, H. (1903) A transpiration model. Roy. Dublin Soc. Sci. Proc. 10, 114–121.
Dixon, H. H. (1914), Transpiration and the Ascent of Sap in Plants, Macmillan and Co., London.
Slatyer, R. O., (1967) Plant-Water Relationships. Academic Press, London.
Noble, P. S. (1999) Physicochemical & Environmental Plant Physiology. 2nd ed. Academic Press, San Diego.
Zimmerman, U., Haase, A., Langbein, D., and Meinzer, F. C. (1993) Mechanisms of long distance water transport in plants: a reexamination of some paradigms in the light of new evidence. Philos. Trans. Roy. Soc. London Ser. B. 341, 19–31.
Canny, M. J. (1998) Transporting water in plants. Am. Sci. 86, 152–159.
Steudle, E. (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Ann. Rev. Plant Physiol. Plant Mol. Biol. 52, 847–875.
Noyes, A. (1900) Die genaue Beziehung zwischen osmotischen Druck und Dampfdruck. Z. Phys. Chem. 35, 707–721.
Herzfeld, K. F. (1937) Thermodynamische und kinetische Betrachtungen über die Zuststandekommen der Dampfdruckerniedrigung von Lösungen. Phys. Z. 38, 58–64.
Duclaux, J. (1965) Théorie de gas. J. Chim. Phys. 65, 435–443.
Hudson, C. S. (1906) The freezing of pure liquids and solutions under various kinds of positive and negative pressure and the similarity between osmotic pressure and negative pressure. Phys. Rev. 22, 257–264.
Renner, O. (1915) Theoretisches und Experimentelles zur Koheäionstheorie der Wasserbewegung. Jahrbücher für wissenschaftliche. Botanik 56, 617–667.
Hammel, H. T. (1995) Roles of colloidal molecules in Starling's hypothesis and in returning interstitial fluid to the vasa recta. Am. J. Physiol. 268, H2133-H2145.
Hammel, H. T. (1991) Internal pressure, hard core and free space volumes and Boltzmann's rule. Phys. Chem. Liq. 23, 69–86.
Hammel, H. T. (1996) Boltzmann's principle depicts distribution of water molecules between vapor and liquid for pure liquid and for aqueous solutions. J. Phys. Chem. 99, 8392–8401.
Hildebrand, J. H. (1928). Internal pressure. In: International Critical Tables of Numerical Data, Physics, Chemistry and Technology, vol. 4. (Washburn, E. W., ed.) Knovel, p. 19.
Soret, Ch. (1884) Arch. Sci. Phys. Nat. 12, 615.
Hammel, H. T. and Maggert, J. E. (1980) Super separation: Soret effect reversed. Separ. Sci. Tech. 15, 81–87.
Schlegel, W. M., Prange, H. D., Furia, E. J., Bowyer, T. D., and Hammel, H. T. (2003) Rethinking the teaching of osmosis. FASEB J. 11, 14.
Hammel, H. T. (2002) Osmotic effects on solvent of solute diffusing in solution. Int. Adv. Res. Physical Chem. 2, 11–33.
Kiil, F. (1982) Kinetics of osmosis. Kidney Int. 21, 303–308.
McKenna, M. J., Heigenhauser, G. J. F., McKelvie, R. S., MacDougal, J. D., and Jones, N. L. (1997) Sprint training enhances ionic regulation during intense exercise in man. J. Physiol. 501, 687–702.
Åstrand, P. O., Rodahl, K., Dahl, H. A., and Strømme, S. R. (2003), Textbook of Work Physiology: Physiological Basis of Exercise 4th ed. Human Kinetics, Champaign, IL.
Starling, E. H. (1896) On the absorption of fluids from connective tissue spaces. J. Physiol. (Lond.) 19, 312–326, 80.
Schmidt-Nielsen, K. (1979) Animal Physiology: Adaptation and Environment. 2nd ed. Cambridge University Press, Cambridge, UK.
Levick, J. R. (2004) Revision of the Starling principle: new views of tissue fluid balance. J. Physiol. (Lond.) 557, 704.
Michel, C. C. (2004) Fluid exchange in the microcirculation. J. Physiol. (Lond.) 557, 701–702.
Adamson, R. H., Lenz, J. F., Zhang, X., Adamson, G. N., Weinbaum, S., and Currie, F. E. (2004) Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J. Physiol. (Lond.) 557, 889–907.
Hammel, H. T. (2004) Ingesting only glucose: behavioral adaptation to lessen high altitude pulmonary edema. Adaptation Biology and Medicine, Volume 4: Current Concepts. (Hargens, A. R., Takeda, N., and Singal, P. K., eds.) Narosa Book distributors Pvt. Ltd., New Delhi, pp. 124–136.
Tripathi, R. C. and Tripathi, B. J. (1984) Anatomy of the eye, orbit, and adnexa, in The Eye. 3rd ed. (Davson, H., ed.). Academic Press, New York.
Davson, H. (1969) The intraocular fluids. The Eye Vol. 1. Ed. H. Academic Press, New York.
Hammel, H. T. (1968) Measurement of turgor pressure and its gradient in the phloem of oak. J. Plant Physiol. 43, 1042–1048.
Münch, E. (1930) Die Stoffbewegungen in der Pflanze. Gustav Fischer Verlagsbuchhandlung, Jena, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hammel, H.T., Schlegel, W.M. Osmosis and solute—Solvent drag. Cell Biochem Biophys 42, 277–345 (2005). https://doi.org/10.1385/CBB:42:3:277
Issue Date:
DOI: https://doi.org/10.1385/CBB:42:3:277