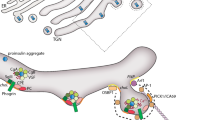
Abstract
Insulin, the major secreted product of the β-cells of the islets of Langerhans, is initially synthesized as a precursor (preproinsulin), from which the mature hormone is excised by a series of proteolytic cleavages. This review provides a personal narrative of some of the key research projects leading to the identification of the central processing enzymes as proprotein convertase 1, proprotein convertase 2, and carboxypeptidase E. It also discusses the central roles of the intragranular environment and chaperone-like proteins in modulating processing activity.
Similar content being viewed by others
References
Ryle, A. P., Sanger, F., Smith, L. F., and Kitai, R. (1955) The disulphide bonds of insulin. Biochem. J. 60, 542–556.
Sures, I., Goeddel, D. V., Gray, A., and Ullrich, A. (1980) Nucleotide sequence of human preproinsulin complementary DNA. Science 208, 57–59.
Ullrich, A., Dull, T. J., Gray, A., Brosius, J., and Sures, I. (1980) Genetic variation in the human insulin gene. Science 209, 612–615.
Dev, I. K. and Ray, P. H. (1990) Signal peptidases and signal peptide hydrolases. J. Bioenerg. Biomembr. 22, 271–290.
Givol, D., De Lorenzo, F., Goldberger, R. F., and Anfinsen, C. B. (1965) Disulfide interchange and the three-dimensional structure of proteins. Proc. Natl. Acad. Sci. USA 53, 676–684.
Steiner, D. F. and Oyer P. E. (1967) The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc. Natl. Acad. Sci. USA 57, 473–480.
Steiner, D. F., Cunningham, D., Spigelman, L., and Aten, B. (1967) Insulin biosynthesis: evidence for a precursor. Science 157, 697–700.
Chance, R. E., Ellis, R. M., and Bromer, W. W. (1968) Porcine proinsulin: characterization and amino acid sequence. Science 161, 165–167.
Nolan, C., Margoliash, E., Peterson, J. D., and Steiner, D. F. (1971) The structure of bovine proinsulin. J. Biol. Chem. 246, 2780–2795.
Clark, J. L., Cho, S., Rubenstein, A. H., and Steiner, D. F. (1969) Isolation of a proinsulin connecting peptide fragment (C-peptide) from bovine and human pancreas. Biochem. Biophys. Res. Commun. 35, 456–461.
Kemmler, W., Peterson, J. D., and Steiner, D. F. (1971) Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J. Biol. Chem. 246, 6786–6791.
Orci, L., Like, A. A., Amherdt, M., Blondel, B., Kanazawa, Y., Marliss, E. B., et al. (1973) Monolayer cell culture of neonatal rat pancreas: an ultrastructural and biochemical study of functioning endocrine cells. J. Ultrastruct. Res. 43, 270–297.
Sorenson, R. L., Steffes, M. W., and Lindall, A. W. (1970) Subcellular localization of proinsulin to insulin conversion in isolated rat islets. Endocrinology 86, 88–96.
Kemmler, W., Steiner, D. F., and Borg, J. (1973) Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J. Biol. Chem. 248, 4544–4551.
Hutton, J. C., Penn, E. J., and Peshavaria, M. (1982) Isolation and characterization of insulin secretory granules from a rat islet cell tumour. Diabetologia 23, 365–373.
Docherty, K. and Hutton, J. C. (1983) Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 162, 137–141.
Hutton, J. C. (1984) Secretory granules. Experientia 40, 1091–1098.
Steiner, D. F., Docherty, K., and Carroll, R. (1984) Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J. Cell. Biochem. 24, 121–130.
Fricker, L. D. and Snyder, S. H. (1982) Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc. Natl. Acad. Sci. USA 79, 3886–3890.
Fricker, L. D., Plummer, T. H., Jr., and Snyder, S. H (1983) Enkephalin convertase: potent, selective, and irreversible inhibitors. Biochem. Biophys. Res. Commun. 111, 994–1000.
Davidson, H. W. (1987) “Enzymes involved in the conversion of proinsulin to insulin in the rat,” PhD thesis, University of Cambridge, Cambridge UK.
Davidson, H. W. and Hutton, J. C. (1987) The insulin-secretory-granule carboxypeptidase H. Purification and demonstration of involvement in proinsulin processing. Biochem. J. 245, 575–582.
Guest, P. C., Ravazzola, M., Davidson, H. W., Orci, L., and Hutton, J. C. (1991) Molecular heterogeneity and cellular localization of carboxypeptidase H in the islets of Langerhans. Endocrinology 129, 734–740.
Steiner, D. F., Hallund, O., Rubenstein, A., Cho, S., and Bayliss, C. (1968) Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes 17, 725–736.
Guest, P. C., Arden, S. D., Rutherford, N. G., and Hutton, J. C. (1995) The post-translational processing and intracellular sorting of carboxypeptidase H in the islets of Langerhans. Mol. Cell. Endocrinol. 113, 99–108.
Fricker, L. D., Evans, C. J., Esch, F. S., and Herbert, E. (1986) Cloning and sequence analysis of cDNA for bovine carboxypeptidase E. Nature 323, 461–464.
Fricker, L. D., Adelman, J. P., Douglass, J., Thompson, R. C., von Strandmann, R. P., and Hutton, J. (1989) Isolation and sequence analysis of cDNA for rat carboxypeptidase E [EC 3.4.17.10], a neuropeptide processing enzyme. Mol. Endocrinol. 3, 666–673.
Varlamov, O. and Fricker, L. D. (1996) The C-terminal region of carboxypeptidase E involved in membrane binding is distinct from the region involved with intracellular routing. J. Biol. Chem. 271, 6077–6083.
Supattapone, S., Fricker, L. D., and Snyder, S. H. (1984) Purification and characterization of a membrane-bound enkephalin-forming carboxypeptidase, “enkephalin convertase.” J. Neurochem. 42, 1017–1023.
Naggert, J. K., Fricker, L. D., Varlamov, O., Nishina, P. M., Rouille, Y., Steiner, D. F., et al. (1995) Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat. Genet. 10, 135–142.
Varlamov, O., Leiter, E. H., and Fricker, L. (1996) Induced and spontaneous mutations at Ser202 of carboxypeptidase E. Effect on enzyme expression, activity, and intracellular routing. J. Biol. Chem. 271, 13981–13986.
Varlamov, O., Fricker, L. D., Furukawa, H., Steiner, D. F., Langley, S. H., and Leiter, E. H. (1997) Beta-cell lines derived from transgenic Cpe(fat)/Cpe(fat) mice are defective in carboxypeptidase E and proinsulin processing. Endocrinology 138, 4883–4892.
Fricker, L. D., Berman, Y. L., Leiter, E. H., and Devi, L. A. (1996) Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J. Biol. Chem. 271, 30619–30924.
Dong, W., Fricker, L. D., and Day, R. (1999) Carboxypeptidase D is a potential candidate to carry out redundant processing functions of carboxypeptidase E based on comparative distribution studies in the rat central nervous system. Neuroscience 89, 1301–1317.
Cool, D. R., Normant, E., Shen, F., Chen, H. C., Pannell, L., Zhang, Y., et al. (1997) Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88, 73–83.
Cool, D. R. and Loh, Y. P. (1998) Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol. Cell. Endocrinol. 139, 7–13.
Irminger, J. C., Verchere, C. B., Meyer, K., and Halban, P. A. (1997) Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J. Biol. Chem. 272, 27532–27534.
Davidson, H. W., Peshavaria, M., and Hutton, J. C. (1987) Proteolytic conversion of proinsulin into insulin. Identification of a Ca2+-dependent acidic endopeptidase in isolated insulin-secretory granules. Biochem. J. 246, 279–286.
Davidson, H.W., Rhodes, C.J., and Hutton, J.C. (1988) Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature 333, 93–96.
Julius, D., Brake, A., Blair, L., Kunisawa, R., and Thorner, J. (1984) Isolation of the putative structural gene for the lysine-arginine — cleaving endopeptidase required for processing of yeast prepro-alpha- factor. Cell 37, 1075–1089.
Thomas, G., Thorne, B. A., Thomas, L., Allen, R. G., Hruby, D. E., Fuller, R., et al. (1988) Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science 241, 226–230.
Fuller, R. S., Brake, A., and Thorner, J. (1989) Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc. Natl. Acad. Sci. U S A 86, 1434–1438.
Smeekens, S. P. and Steiner, D. F. (1990) Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J. Biol. Chem. 265, 2997–3000.
Smeekens, S. P., Avruch, A. S., LaMendola, J., Chan, S. J., and Steiner, D. F. (1991) Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc. Natl. Acad. Sci. U S A 88, 340–344.
Seidah, N. G., Marcinkiewicz, M., Benjannet, S., Gaspar, L., Beaubien, G., Mattei, M. G., et al. (1991) Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol. Endocrinol. 5, 111–122.
Thomas, L., Leduc, R., Thorne, B. A., Smeekens, S. P., Steiner, D. F., and Thomas, G. (1991) Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc. Natl. Acad. Sci. U S A 88, 5297–5301.
Bailyes, E. M., Shennan, K. I., Seal, A. J., Smeekens, S. P., Steiner, D. F., Hutton J. C., et al. (1992) A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem. J. 285, 391–394.
Bennett, D. L., Bailyes, E. M., Nielsen, E., Guest, P. C., Rutherford, N. G., Arden, S. D., et al. (1992) Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J. Biol. Chem. 267, 15229–15236.
Smeekens, S. P., Montag, A. G., Thomas, G., Albiges-Rizo, C., Carroll, R., Benig, M., et al. (1992) Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc. Natl. Acad. Sci. USA 89, 8822–8826.
Muller, L. and Lindberg, I. (1999) The cell biology of the prohormone convertases PC1 and PC2. Prog. Nucleic Acid Res. Mol. Biol. 63, 69–108.
Zhou, A. and Mains, R. E. (1994) Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J. Biol. Chem. 269, 17440–17447.
Goodman, L. J. and Gorman, C. M. (1994) Autoproteolytic activation of the mouse prohormone convertase mPC1. Biochem. Biophys. Res. Commun. 201, 795–804.
Seidah, N. G. and Chretien, M. (1999) Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 848, 45–62.
Alarcon, C., Lincoln, B., and Rhodes, C. J. (1993) The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J. Biol. Chem. 268, 4276–4280.
Guest, P. C., Rhodes, C. J., Hutton, J. C. (1989) Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem. J. 257, 431–437.
Zhu, X., Orci, L., Carroll, R., Norrbom, C., Ravazzola, M., and Steiner, D. F. (2002) Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc. Natl. Acad. Sci. USA 99, 10299–10304.
Zhu, X., Zhou, A., Dey, A., Norrbom, C., Carroll, R., Zhang, C., et al. (2002) Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl. Acad. Sci. USA 99, 10293–10298.
Jackson, R. S., Creemers, J. W., Ohagi, S., Raffin-Sanson, M. L., Sanders, L., Montague C. T., et al. (1997) Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16, 303–306.
Guest, P. C., Arden, S. D., Bennett, D. L., Clark, A., Rutherford, N. G., and Hutton, J. C. (1992) The post-translational processing and intracellular sorting of PC2 in the islets of Langerhans. J. Biol. Chem. 267, 22401–22406.
Lamango, N. S., Apletalina, E., Liu, J., and Lindberg, I. (1999) The proteolytic maturation of prohormone convertase 2 (PC2) is a pH- driven process. Arch. Biochem. Biophys. 362, 275–282.
Creemers, J. W., Usac, E. F., Bright, N. A., Van de Loo, J. W., Jansen E., Van de Ven, W. J., et al. (1996) Identification of a transferable sorting domain for the regulated pathway in the prohormone convertase PC2. J. Biol. Chem. 271, 25284–25291.
Hsi, K. L., Seidah N. G., De Serres, G., and Chretien, M. (1982) Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett. 147, 261–266.
Braks, J. A. and Martens, G. J. (1994) 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell 78, 263–273.
Shen, F. S., Seidah, N. G., and Lindberg, I. (1993) Biosynthesis of the prohormone convertase PC2 in Chinese hamster ovary cells and in rat insulinoma cells. J. Biol. Chem. 268, 24910–24915.
Zhu, X. and Lindberg, I. (1995) 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J. Cell. Biol. 129, 1641–1650.
Westphal, C. H., Muller, L., Zhou, A., Zhu, X., Bonner-Weir, S., Schambelan, M., et al. (1999) The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell 96, 689–700.
Bailyes, E. M., Shennan, K.I., Usac, E. F., Arden, S. D., Guest, P. C., Docherty, K., et al. (1995) Differences between the catalytic properties of recombinant human PC2 and endogenous rat PC2. Biochem. J. 309, 587–594.
Martens, G. J., Braks, J.A., Eib, D. W., Zhou, Y., and Lindberg, I. (1994) The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc. Natl. Acad. Sci. U S A 91, 5784–5787.
Zhu, X., Rouille, Y., Lamango, N. S., Steiner, D. F., and Lindberg, I. (1996) Internal cleavage of the inhibitory 7B2 carboxyl-terminal peptide by PC2: a potential mechanism for its inactivation. Proc. Natl. Acad. Sci. USA 93, 4919–4924.
Fortenberry, Y., Hwang, J. R., Apletalina, E. V., and Lindberg, I. (2002) Functional characterization of ProSAAS: similarities and differences with 7B2. J. Biol. Chem. 277, 5175–5186.
Berman, Y., Mzhavia, N., Polonskaia, A., and Devi, L. A. (2001) Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J. Biol. Chem. 276, 1466–1473.
Furuta, M., Carroll, R., Martin, S., Swift, H. H., Ravazzola, M., Orci, L., et al. (1998) Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J. Biol. Chem. 273, 3431–3437.
Lipkind, G. and Steiner, D. F. (1999) Predicted structural alterations in proinsulin during its interactions with prohormone convertases. Biochemistry 38, 890–896.
Docherty, K., Carroll, R. J., and Steiner, D. F. (1982) Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc. Natl. Acad. Sci. USA 79, 4613–4617.
Rhodes, C. J., Lincoln, B., and Shoelson, S. E. (1992) Preferential cleavage of des-31,32-proinsulin over intact proinsulin by the insulin secretory granule type II endopeptidase. Implication of a favored route for prohormone processing. J. Biol. Chem. 267, 22719–22727.
Demaurex, N., Furuya, W., D’Souza, S., Bonifacino, J. S., and Grinstein, S. (1998) Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273, 2044–2051.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidson, H.W. (Pro)Insulin processing. Cell Biochem Biophys 40 (Suppl 3), 143–157 (2004). https://doi.org/10.1385/CBB:40:3:143
Issue Date:
DOI: https://doi.org/10.1385/CBB:40:3:143