Abstract
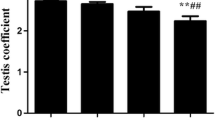
Early studies on nickel essentiality with rats and goats indicated that nickel deprivation impaired reproductive performance. Nickel also has been found to influence cyclic nucleotide gated channels (CNG); these types of channels are important in sperm physiology. Thus, two experiments were conducted to test the hypothesis that nickel deficiency affects sperm physiology in a manner consistent with nickel having an essential function related to CNG channel functions. The experiments were factorially arranged with four treatment groups of eight weanling rats in each. In experiment 1, the treatments were supplemental dietary nickel of 0 and 1 mg/kg and N ω-nitro-l-arginine methyl ester (l-NAME, a nitric oxide synthase inhibitor) added to the drinking water (50 mg/100 mL) the last 3 wk of an 8-wk experiment. In experment 2, the treatments were supplemental dietary nickel at 0 and 1 mg/kg and supplemental dietary sodium chloride (NaCl) at 0 and 80 g/kg. The NaCl and l-NAME variables were included to act as stressors affecting CNG channel activity. The basal diet contained per kilogram about 27 µg of nickel and 1 g of sodium. After 8 wk in experiment 1 and 16 wk in experiment 2, urine while fasting and testes and epididymis in both experiments, and seminal vesicles and prostates in experiment 2 were harvested for analysis. Nickel deprivation significantly decreased spermatozoa motility and density in the epididymides, epididymal transit time of spermatozoa, and testes sperm production rate. Nickel deficiency also significantly decreased the weights of the seminal vesicles and prostate glands. Excessive NaCl had no effect on sperm physiology; however, it decreased prostate gland weights. The findings support the hypothesis that nickel has an essential function that possibly could affect reproductive performance in higher animals, perhaps through affecting a CNG channel function.
Similar content being viewed by others
References
F. H. Nielsen, D. R. Myron, S. H. Givand, et al., Nickel deficiency in rats, J. Nutr. 105, 1620–1630 (1975).
M. Anke, B. Groppel, U. Krause, et al., Further data on the biological essentiality of nickel, in Trace Elements in Man and Animals 6, L. S. Hurley, C. L. Keen, B. Lonnerdal, et al., eds., Plenum, New York, pp. 467–469 (1988).
M. Ildefonse and N. Bennett, Single-channel study of the cGMP-dependent conductance of retinal rods from incorporation of native vesicles into planar bilayers, J. Membr. Biol. 123, 133–147 (1991).
M. Ildefonse, S. Crouzy, and N. Bennett, Gating of retinal rod cation channel by different nucleotides: comparative study of unitary currents, J. Membr. Biol. 130, 91–104 (1992).
J. W. Karpen, R. L. Brown, L. Stryer, et al., Interaction between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods, J. Gen. Physiol. 101, 1–25 (1993).
P. A. Kingston, F. Zufall, and C. J. Barnstable, Rat hippocampal neurons express genes for both rod retinal and olfactory cyclic nucleotide-gated channels: novel targets for cAMP/cGMP function, Proc. Natl. Acad. Sci. USA 93, 10,440–10,445 (1996).
J. Bradley, Y. Zhang, R. Bakin, et al., Functional expression of the heteomeric “olfactory” cyclic nucleotide-gated channel in the hippocampus: a potential effector of synaptic plasticity in brain neurons, J. Neurosci. 17, 1993–2005 (1997).
I. Ahmad, C. Korbmacher, A. S. Segal, et al., Mouse cortical collecting duct cells show nonselective cation channel activity and express a gene related to the cGMP-gated rod photoreceptor channel, Proc. Natl. Acad. Sci. USA 89, 10,262–10,266 (1999).
M. Biel, X. Zong, M. Distler, et al., Another member of the cyclic nucleotide-gated channel family, expressed in testis, kidney, and heart, Proc. Natl. Acad. Sci. USA 91, 3505–3509 (1994).
M. Biel, W. Altenhofen, R. Hullin, et al., Primary structure and functional expression of a cyclic nucleotide-gated channel from rabbit aorta, FEBS Lett. 329, 134–138 (1993).
I. Weyand, M. Godde, S. Frings, et al., Cloning and functional expression of a cyclicnucleotide-gated channel from mammalian sperm, Nature 368, 859–863 (1994).
B. Wiesner, J. Weiner, R. Middendorff, et al., Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm, J. Cell Biol. 142, 473–484 (1998).
D. Ritter, A. D. Dean, S. L. Gluck, et al., Natriuretic peptide receptors A and B have different cellular distribution in rat kidney, Kidney Int. 48, 5758–5766 (1995).
Y. Terada, K. Tomita, H. Nonoguchi, et al., PCR localization of C-type natriuretic peptide and B-type receptor mRNAs in rat nephron segments, Am. J. Physiol. 267, F215–F222 (1994).
J. Navarro, A. Sanchez, J. Saiz, et al., Hormonal, renal, and metabolic alterations during hypertension induced by chronic inhibition of NO in rats, Am. J. Physiol. 267, R1516–R1521 (1994).
P. Burguera, A. Sanchez de Briceno, C. E. Rondon, et al., Determination of nickel in saliva by electrothermal atomic absorption spectrometry using various chemical modifiers with Zeeman effect background correction, J. Trace Elements Med. Biol. 12, 115–120 (1998).
R. L. Dahlquist and J. W. Knoll, Inductively coupled plasma-atomic emission spectrometry: analysis of biological materials and soils in major, trace and ultra-trace elements, Appl. Spectrosc. 32, 1–29 (1978).
P. G. Reeves and K. L. Rossow, Zinc deficiency affects the activity and protein concentration of angiotensin-converting enzyme in rat testes, Proc. Soc. Exp. Biol. Med. 203, 336–342 (1993).
G. W. Robb, R. P. Amann, and G. J. Killian, Daily sperm production and epididymal sperm reserves of pubertal and adult rats, J. Reprod. Fertil. 54, 103–107 (1978).
A. Fernandez-Rivas, J. Garcia-Estan, and F. Vargas, Effects of chronic increased salt intake on nitric oxide synthesis inhibition-induced hypertension, J. Hypertens. 13, 123–128 (1995).
A. Swislocki, T. Eason, and C. A. Kaysen, Oral administration of the nitric oxide biosynthesis inhititor, N-nitro-l-arginine methyl ester (l-NAME), causes hypertension, but not glucose intolerance or insulin resistance, in rats, Am. J. Hypertens. 8, 1009–1014 (1995).
H. Tsukahara, T. Imura, S. Tsuchida, et al., Renal functional measurements in young rats with chronic inhibition of nitric oxide synthase, Acta Paediatr. Jpn. 38, 614–618 (1996).
S. L. Lubarsky, R. A. Ahokas, S. A. Friedman, et al., The effect of chronic nitric oxide synthesis inhibition on blood pressure and angiotension II responsiveness in the pregnant rat, Am. J. Obstet. Gynecol. 176, 1069–1076 (1997).
N. Akuzawa, T. Nakamura, T. Kurashina, et al., Antihypertensive agents prevent nephrosclerosis and left ventricular hypertrophy induced in rats by prolonged inhibition of nitric oxide synthesis, Am. J. Hypertens. 11, 697–707 (1998).
R. Kakela, A. Kakela, and H. Hyvarinen, Effects of nickel chloride on reproduction of the rat and possible antagonistic role of selenium, Comp. Biochem. Physiol. C. 123, 27–37 (1999).
E. Obone, S. K. Chakrabarti, C. Bai, et al., Toxicity and bioaccumulation of nickel sulfate in Sprague-Dawley rats following 13 weeks of subchronic exposure, J. Toxicol. Environ. Health 57A, 379–401 (1999).
L. M. King, W. A. Banks, and W. J. George, Differential zinc transport into testis and brain of cadmium-sensitive and -resistant murine strains, J. Androl. 21, 656–663 (2000).
S. E. Gordon and W. N. Zagotta, Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels, Proc. Natl. Acad. Sci. USA 92, 10,222–10,226 (1995).
B. Morton, J. Harrigan-Lum, L. Albagli, et al., The activation of motility in quiescent hamster sperm from the epididymis by calcium and cyclic nucleotides, Biochem. Biophys. Res. Commun. 56, 372–379 (1974).
R. A. Anderson, Jr., K. A. Feathergill, R. G. Rawlins, et al., Atrial natriuretic peptide: a chemoattractant of human spermatozoa by a guanylate cyclase-dependent pathway, Mol. Reprod. Dev. 40, 371–378 (1995).
D. E. McCoy, S. E. Guggino, and B. A. Stanton, The renal cGMP-gated cation channel: its molecular structure and physiological role, Kidney Int. 48, 1125–1133 (1995).
D. H. Vandorpe, F. Ciampolillo, R. B. Green, et al., Cyclic nucleotide-gated cation channels mediate sodium absorption by IMCD (mIMCD-K2) cells, Am. J. Physiol. 272, C901–C910 (1997).
J. C. Hansen and R. C. Jones, In vivo microperfusion of the ductuli efferentes testis of the rat: flow dependence of fluid reabsorption, Exp. Physiol. 81, 633–644 (1996).
R. Middendorff, M. S. Davidoff, S. Behrends, et al., Multiple roles of the messenger molecule cGMP in testicular function, Andrologia 32, 55–59 (2000).
R. Middendorff, D. Muller, H. J. Paust, et al., New aspects of Leydig cell function, Adv. Exp. Med. Biol. 424, 125–138 (1997).
P. Rossi, R. Pezzotti, M. Conti, et al., Cyclic nucleotide phosphodiesterases in somatic and germ cells of mouse seminiferous tubules, J. Reprod. Fertil. 74, 317–322 (1985).
Author information
Authors and Affiliations
Additional information
Part of the data was presented at the Experimental Biology 2001 Meeting, Orlando, FL, March 31–April 4, 2001. (F. H. Nielsen, E. O. Uthus and K. Yokoi, Dietary nickel deprivation decreases sperm motility and evokes hypertension in rats, FASEB J. 15, A972 (2001), and K. Yokoi, E. O. Uthus and F. H. Nielsen, Nickel deficiency induces renal damages and hypertension in rats which is augmented by sodium chloride, FASEB J. 15, A973 (2001).
The US Department of Agriculture, Agricultural Research Service, Northern Plains Area, is an equal opportunity/affirmative action employer and all agency services are available without discrimination.
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply its approval to the exclusion of the products that may also be suitable.
Rights and permissions
About this article
Cite this article
Yokoi, K., Uthus, E.O. & Nielsen, F.H. Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93, 141–153 (2003). https://doi.org/10.1385/BTER:93:1-3:141
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:93:1-3:141