Abstract
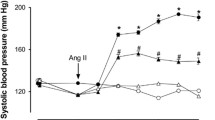
Because zinc attenuates endothelial cell dysfunction that proceeds atherosclerosis, depressed zinc status may be involved in the initiation of endothelial dysfunction. However, before recommending a zinc-enriched diet to reduce the risks for atherosclerosis, the effect of excess zinc on endothelial cell functions in normozincemic status should be known. Therefore, in this study, the effect of dietary zinc on normal endothelial cell functions in animals subjected to a diet containing 334±58 ppm zinc for 30 d was studied to see whether supplemented zinc has an effect on endothelial cells. Despite a slight increase in blood zinc, unaltered aortic and kidney zinc contents were associated with unchanged blood pressure in rats subjected to a zinc-enriched diet. Increased basal nitric oxide and prostacyclin were accompanied by a normal response to phenylephrine. Dietary zinc influenced neither endothelial-dependent nor endothelial-independent relaxations significantly. However, it elevated the share of M1-type cholinoceptor response as well as dilator prostaglandin release, which seems to be nitric oxide dependent. There was a strong correlation (r=0.826, p<0.05) between M1-type cholinoceptor response and prostacyclin release in zinc-treated rings. These results suggested that zinc ions increases M1-mediated prostacyclin release in normal endothelial cells without altering intracellular pathways.
Similar content being viewed by others
References
T. E. Tuormae, The adverse effect of zinc deficiency, J. Orthomol. Med. 10, 149–164 (1995).
B. Hennig, M. Toborek, and C. J. McClain, Antiatherogenic properties of zinc implications in endothelial cell metabolism, Nutrition 12, 711–717 (1996).
C. J. McClain, P. Morris, and B. Hennig, Zinc and endothelial function, Nutrition 11, 117–120 (1995).
B. Hennig, Y. Wang, S. Ramasamy, et al., Zinc protects against tumor necrosis factorinduced disruption of porcine endothelial cell monolayer integrity, J. Nutr. 123, 1003–1009 (1993).
B. Hennig, P. Meerarani, M. Toborek, et al., Antioxidant like properties of zinc in activated endothelial cells, J. Am. Coll. Nutr. 18, 152–158 (1999).
G. Oner, I. Bilgen, M. Edremitlioglu, et al., The effect of cadmium and zinc ions on vascular tonus, in Metal Ions in Biology and Medicine, Vol. 6, J. A. Centeno, P. Collery, G. Vernet, et al., eds., John Libbey Eurotext, Paris, pp. 638–640 (2000).
J. B. Smith, S. D. Dwyer, and L. Smith, Cadmium evokes inositol phosphate formation and calcium mobilization. Evidence for cell surface receptor that cadmium stimulate and zinc antagonizes, J. Biol. Chem. 264, 7115–7118 (1989).
J. Benters, U. Flogel, T. Schafer, et al., Study of the interactions of cadmium and zinc ions with cellular calcium homeostasis using 19F-NMR spectroscopy, J. Biochem. 322, 793–799 (1997).
R. Diaz-Arrastia and E. Hashemi, Zinc and ascorbic acid coordinately promote lipid peroxidation in brain membranes, J. Mol. Neurosci. 14, 167–173 (2000).
L. S. Prothero, A. Mathie, and C. D. Richards, Purinergic and muscarinic receptor activation activates a common calcium entry pathway in rat neurons and glial cells, Neuropharmacology 39, 1768–1778 (2000).
R. A. Shapiro, N. M. Scherer, B. A. Habacker, et al., Isolation sequence and functional expression of the mouse M1 muscarinic Ach receptor gene, J. Biol. Chem. 263, 18,397–18,403 (1988).
Z. L. Lu and E. C. Hulme, A network of conserved intramolecular contacts defines the off-stage of the transmembrane switch mechanism in a seven transmembrane receptor, J. Biol. Chem. 275, 5682–5686 (2000).
M. P. Demontis, M. V. Varoni, A. R. Volpe, et al., Role of nitric oxide synthase inhibition in the acute hypertensive response to intracerebroventricular cadmium, Br. J. Pharmacol. 123, 129–135 (1998).
D. C. Ramirez, L. D. Martinez, E. Marchevsky, et al., Biphasic effect of cadmium in noncytotoxic conditions on the secretion of nitric oxide from peritoneal macrophages, Toxicology 139, 167–177 (1999).
S. Taddei, A. Virdis, L. Ghiadoni, et al., Endothelial dysfunction in hypertension, J. Nephrol. 13, 205–210 (2000).
O. L. Woodman, O. Wongsawatkul, and C. G. Sobey, Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries, Clin. Exp. Pharmacol. Physiol. 27, 34–40 (2000).
Y. Nishikawa, D. W. Stepp, and W. M. Chilian, In vivo location and mechanism of EDHF-mediated vasodilatation in canine coronary microcirculation, Am. J. Physiol. 277, H1252-H1259 (1999).
G. Öner and I. Bilgen, l-Arginine induced-changes the characteristics of endothelial relaxants, J. Basic Clin. Physiol. Pharmacol. 12, 77–90 (2001).
D. Salvemini, S. L. Settle, J. L. Masferrer, et al., Regulation of prostaglandin production by nitric oxide; an in vivo analysis, Br. J. Pharmacol. 114, 1171–1778 (1995).
D. Salvemini, M. G. Currie, and V. Mallace, Nitric oxide mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrosodilators, J. Clin. Invest. 97, 2562–2568 (1996).
H. F. Cheng, J. L. Wang, M. Z. Zhang, et al., Nitric oxide regulates renal cortical cyclooxygenase-2 expression, Am. J. Physiol. (Renal Physiol.) 278, F122-F129 (2000).
E. N. Bakker and P. Sipkema, Permissive effect of nitric oxide in arachidonic acid induced dilation in isolated rat arterioles, Cardiovasc. Res. 38, 782–787 (1998).
O. M. Marita, Y. Mano, and S. Murota, Differential effects of nitric oxide on the activity of prostaglandin endoperoxide H synthase-1 and -2 in vascular endothelial cells, Prostaglandins Leukotrienes Essential Fatty Acids 62, 161–167 (2000).
D. Salvemini, T. P. Misko, J. L. Masferrer, et al., Nitric oxide activates cyclooxygenase enzymes, Proc. Natl. Acad. Sci. USA 90, 7240–7244 (1993).
J. X. Chen, L. C. Berry, Jr., B. W. Christhman, et al., No regulates LPS-stimulated cyclooxygenase gene expression and activity in pulmonary artery endothelium, Am. J. Physiol. (Lung Cell Mol. Physiol.) 280, L450-L457 (2001).
T. Osanai, N. Fujita, N. Fujiwara, et al., Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells, Am. J. Physiol. (Heart Circ. Physiol.) 278, H233-H238 (2000).
O. Kasonen, H. Kankaanranta, U. Mala-Ranta, et al., Inhibition by nitric oxide releasing compounds of prostacyclin production in human endothelial cells, Br. J. Pharmacol. 125, 247–254 (1998).
L. Smith, V. Pijuan, Y. Zhuang, et al., Reversible desensitization of fibroblast to cadmium receptor stimuli: evidence that growth in high zinc represses a xenobiotic receptor, Exp. Cell Res. 202, 174–182 (1992).
J. Garcia-Colunga, M. Gonzales-Herrera, and R. Miledi, Modulation of alpha 2 beta 4 neuronal nicotinic acetylcholinic receptors by zinc, Neuroreport 12, 147–150 (2001).
Y. Nasa, H. Kume, and S. Takeo, Acethylcholine induced vasoconstrictor response of coronary vessels in rats: a possible contribution of M2 muscarinic receptor activation, Heart Vessels 12, 179–191 (1997).
M. G. Tyagi, H. Kan, Y. Ruan, et al., Studies on the characterization of the subtype(s) of muscarinic receptor involved in prostacyclin synthesis in rabbit cardiomyocytes, J. Recept. Signal Transduct. Res. 16, 273–296 (1996).
M. E. Murphy and J. E. Brayden, Apamin sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries, J. Physiol. (Lond.) 489, 723–734 (1995).
K. Komori and P. M. Vanhoutte, Endothelium derived hyperpolarizing factor, Blood Vessels 27, 238–245 (1990).
H. I. Chen and Y. L. Liao, Effect of chronic exercise on muscarinic receptor-mediated vasodilatation in rats, Chin. J. Physiol. 41, 161–166 (1998).
U. Schönbeck, G. K. Sukhova, P. Graber, et al., Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions, Am. J. Pathol. 155, 1281–1291 (1999).
M. P. Gupta, M. D. Ober, C. Patterson, et al., Nitric oxide attenuates H(2)O(2)-induced endothelial barrier dysfunction: mechanisms of protection, Am. J. Physiol. (Lung Cell Mol. Physiol.) 280, L116-L126 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oner, G., Bilgen, I., Edremitlioglu, M. et al. Dietary zinc modifies the characteristics of endothelial dilation in normozincemic rats. Biol Trace Elem Res 92, 123–137 (2003). https://doi.org/10.1385/BTER:92:2:123
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:92:2:123