Abstract
The activity of glutathione peroxidase (GSH-Px), serum selenium (Se), and thiobarbituric acid reactive substances (TBARS) were measured in the whole blood of 148 healthy adults aged 20–60 yr from the fishing and rural communities of “Rabo de Peixe,” The Azores, Portugal.
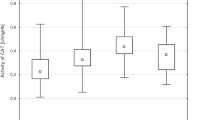
The subjects did not live in the same household and had different socioeconomic profiles and dietary habits. The serum lipid profile and selected life habits were also considered in this study. No significant differences in the activity of GSH-Px were found in the interpopulation or intrapopulation analyses, classified by age or lipid profile.
An age-dependent GSH-Px increase was noted in the younger male (M) subgroups (20–39 yr). The Se levels were higher in fishers (f) of both genders (M, F) than in subjects living in the rural (r) environment: 110±25 µg/L (f, M), 89±20 µg/L (f, F), 88±22 µg/L (r, M) and 80±17 µg/L (r, F). In the fishers, but not in the rural population, Se was higher in the males, but it did not show significant variation with age. The levels of TBARS were lower in the f than in the r male group. The Se level was lower and TBARS higher in the hyperlipemic women in the f group, compared to the corresponding controls.
Our results suggest that the fishers (mainly men) show a better antioxidant status than that of their rural counterparts, due to differences in dietary habits between the study populations and between genders.
Similar content being viewed by others
References
D. Behne, C. Weiss-Nowak, H. Gessner, and A. Kyriakopoulos, New mammalian selenoproteins, in Trace Elements in Medicine, Health and Atherosclerosis, M. F. Reis, J. M. Pereira Miguel, AAS.C. Machado, and M. Abdulla, eds., Smith-Gordon, Nishimura, pp. 91–96 (1995).
H. Robberecht and H. Deelstra, Factors influencing blood selenium concentration values: a literature review, J. Trace Elements Electrolytes Health Dis. 8, 129–143 (1994).
K. R. Maddipati and L. J. Marnett, Characterization of the major hydroperoxide-reducing activity of human plasma, J. Biol. Chem. 262(36), 17,398–17,403 (1987).
M. Björnstedt, J. Xue, W. Huang, B. Akesson, and A. Holmgren, The thioredoxin and glutaredoxinsystems are efficient electron donors to human plasma glutathione peroxidase, J. Biol. Chem. 269, 29,382–29,384 (1994).
R. J. Kulmacz and W. E. M. Lands, Characteristics of prostaglandin II synthase, in Advances in Prostaglandin, Tromboxane, and Leukotriene Research, Volume 11, B. Samuelsson, R. Paoletti, and R. Ramwell, eds., Raven, New York, pp. 93–95 (1983)
H. Imai, H. Kashiwazaki, T. Suzuki, et al., Selenium levels and glutathione peroxidase activities in blood in an Andean high-altitude population, J. Nutr. Sci. Vitaminol. 41, 349–361 (1995).
B. P. Yu, Cellular defenses against damage from reactive oxygen species, Physiol. Rev. 74(1), 139–162 (1994).
H. Wiseman and B. Halliwell, Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer, Biochem. J. 313, 17–29 (1996).
J. Nève, Selenium as a risk factor for cardiovascular diseases, J. Cardiovasc. Risk 3, 42–46 (1996).
J. Nève, S. Chamart, and L. Molle, Optimization of a direct procedure for determination of selenium in plasma and erythrocytes using Zeeman effect atomic absorption spectroscopy, in Trace Element Analytical Chemistry in Medicine and Biology, Volume 4, P. Bratter and P. Schramel, eds., Walter de Gruyter, New York, pp. 349–358 (1987).
D. E. Paglia and W. N. Valentine, Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase, J. Lab. Clin. Med. 70, 158–169 (1967).
K. Satoh, Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method, Clin. Chim. Acta 90, 37–43 (1978).
W. Wasowicz, J. Nève, and A. Peretz, Optimized steps in fluorimetric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage, Clin. Chem. 39, 12,2522–2526 (1993).
L. I. Guidez, G. J. Miller, M. Burstein, S. Slage, and H. A. Eder, Separation and quantification of subclasses of human plasma HDL by a simple precipitation procedure, J. Lipid Res. 23, 1206–1223 (1982).
A. M. Viegas-Crespo, M. L. Pavão, M. L. Mira, I. Torres, M. J. Halpern, and J. Nève, Comparison of serum selenium levels in inhabitants from different portuguese regions, in Therapeutic Uses of Trace Elements: Status, Epidemiology of Trace Elements and Intervention Studies, J. Nève, P. Chappuis, and M. Lamand, eds., Plenum, New York, pp. 351–354 (1996).
A. M. Viegas-Crespo, J. Nève, M. L. Monteiro, M. F. Amorim, O. Paulo, and M. J. Halpern, Selenium and lipid parameters in plasma of portuguese subjects, J. Trace Elements Electrolytes Health Dis. 8, 119–122 (1994).
A. M. Viegas-Crespo, M. L. Pavão, O. Paulo, V. Santos, M. C. Santos, and J. Nève, Trace element status (Se, Cu, Zn) and serum lipid profile in portuguese subjects of San Miguel Island from Azores’ archipelago, J. Trace Elements Med. Biol. 14, 1–5 (2000).
L. Hagmar, M. Persson-Moschos, B. Akesson, and A. Schutz, Plasma levels of selenium, selenoprotein P and glutathione peroxidase and their correlations to fish intake and serum levels of thyrotropin and thyroid hormones: a study on Latvian fish consumers, Eur. J. Nutr. 52(11), 796–800 (1998).
H. Robberecht, P. Hendrix, R. Van Cauwenbergh, and H. Deelstra, Actual daily dietary intake of selenium in Belgium, using duplicate portion sampling, Z. Lebensm. Unters. Forsch. 199, 251–254 (1994).
A. M. Viegas-Crespo, M. L. Pavão, V. Santos, et al., Selenium status and cardiovascular risk factors in populations from different portuguese regions, in Natural Antioxidants and Food Quality in Atherosclerosis and Cancer Prevention: Selenium Intake and Status of Various Populations, J. Kumpulainen and J. Salonen, eds., The Royal Society of Chemistry, Cambridge, pp. 188–194 (1996).
J. Versieck and R. Cornelis, Trace elements in Plasma or Serum, CRC, Boca Raton, FL. (1989).
L. Guemouri, Y. Artur, B. Herbeth, C. Jeandel, G. Cuny, and G. Siest, Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood, Clin. Chem. 37(11), 1932–1937 (1991).
C. Berr, A. Nicole, J. Godin, et al., Selenium and oxygen-metabolizing enzymes in elderly community residents: a pilot epidemiological study, J. Am. Geriatr. Soc. 41(2), 143–148 (1993).
F. Girodon, D. Blache, A.-L. Monget, et al., Effect of a two-year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters, J. Am. Coll. Nutr. 16(4), 357–365 (1997).
I. Ceballos-Picot, J. M. Trivier, A. Nicole, P. M. Sinet, and M. Thevenin, Age-correlated modifications of copper-zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes, Clin. Chem. 38, 66–70 (1992).
R. E. Pinto and W. Bartley, The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates, Biochem. J. 112, 109–114 (1969).
J. Köhrle, R. Brigelius-Flohé, A. Böck, R. Gärtner, O. Meyer, and L. Flohé, Selenium in biology: facts and medical perspectives, Biol. Chem. 381, 849–864 (2000).
L. Flohé, E. Wingender, and R. Brigelius-Flohé, Regulation of glutathione peroxidases, in Oxidative Stress and Signal Transduction, H. J. Forman and E. Cadenas, eds., Chapman & Hall, New York, pp. 415–440 (1997).
R. Brigelius-Flohé, Tissue-specific functions of individual glutathione peroxidases, Free Radical Biol. Med. 27(9/10), 951–965 (1999).
T. Fox, C. Atherton, S. Fairweather-Tait, et al., Changes in indices of selenium status in men on low, medium, and high intakes, in Trace Elements in Man and Animals 10, A. M. Roussel, R. A. Andersen, and A. E. Favier, eds., Kluver Academic/Plenum Ps, New York, pp. 877–881 (2000).
D. Harman, Free radical theory of aging: history, in Free Radicals and Aging, I. Emerit and B. Chance, eds., Birkhauser, Bazel, pp. 1–10 (1992).
H. R. Andersen, J. B. Nielsen, F. Nielsen, and P. Grandjean, Antioxidative enzyme activities in human erythrocytes, Clin. Chem. 43(4), 562–568 (1997).
H.-K. Wong, J. Riondel, and A. Favier, Biomarkers of mouse aging: Modifications of minerals on antioxidant enzymes, in Trace Elements in Man and Animals 10, A. M. Roussel, R. A. Andersen, and A. E. Favier, eds., Kluver Academic/Plenum Ps, New York, p. 448 (2000).
Y. Ito, O. Kajkenova, R. J. Feuers, et al., Impaired glutathione peroxidase activity accounts for the age-related accumulation of hydrogen peroxide in activated human neutrophils, J. Gerontol. A: Biol. Sci. Med. Sci. 53(3), M169-M175 (1998).
Y. Rayssiguier and A. Mazur, Trace elements: metabolism and oxidative modifications of lipoproteins, in Trace Elements in Man and Animals 10, A. M. Roussel, R. A. Andersen, and A. E. Favier, eds., Kluver Academic/Plenum Ps, New York, pp. 97–103 (2000).
J. T. Salonen, R. Salonen, K. Seppaenen, et al., Relationship of serum selenium and antioxidants to plasma lipoproteins, platelet aggregability and prevalent ischaemic heart disease in Eastern Finnish men, Atherosclerosis 70, 155–165 (1988).
S. G. F. Bukkens, N. de Vos, F. J. Kok, E. G. Schouten, A. M. Bruijin, and A. Holfman, Selenium status and cardiovascular risk factors in healthy Dutch subjects, J. Am. Coll. Nutr. 9(2), 128–135 (1990).
M. L. Pavão, V. Santos, A. Costa, et al., Selenium, copper and zinc in some Azorean populations, in New Aspects of Trace Element Research, M. Abdulla, M. Bost, S. Gamon, P. Arnaud, and G. Chazot, eds., Smith-Gordon, London, pp. 42–44 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pavão, M.L., Cordeiro, C., Costa, A. et al. Comparison of whole-blood glutathione peroxidase activity, levels of serum selenium, and lipid peroxidation in subjects from the fishing and rural communities of “Rabo de Peixe” village, San Miguel Island, the Azores’ Archipelago, Protugal. Biol Trace Elem Res 92, 27–40 (2003). https://doi.org/10.1385/BTER:92:1:27
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1385/BTER:92:1:27