Abstract
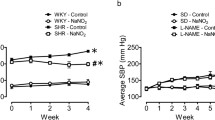
Because zinc (Zn) is an important component for cell protection against certain oxygen species, it has been suggested that Zn deficiency impairs the potent oxidant defense capacity, which is constitutively provided in the vascular system. However, the influence of dietary Zn deficiency on systemic blood pressure and vascular system is controversial and unclear. We therefore examine the effect of dietary Zn deficiency on systemic blood pressure, a potent superoxide scavenger, aortic Cu/Zn superoxide dismutase (SOD) activity, a most representative synthase of the endothelium-derived relaxing factor, and aortic endothelial nitric oxide synthase (eNOS) expression. Furthermore, the direct effects of intravenous administration of NOS inhibitor, N ω-nitro-l-arginine methyl ester (l-NAME), and a SOD mimetic compound, tempol, in normotensives were tested in Wistar-Kyoto (WKY) rats. A Zn-deficient diet (4 wk) contributed to growth retardation, the decrease in thymus weight, and the lower levels of serum Zn compared with the standard diet group. However, no significant difference in conscious systolic and diastolic blood pressure was found in the Zn-deficiency group. The administration of l-NAME caused an increase in the mean arterial pressure (MAP) levels in the two groups of rats and the involvement of the vasodilator nitric oxide (NO) in the regulation of systemic BP in the normotensive state. On the other hand, administration of the superoxide scavenger, tempol, led to a decrease in MAP levels in the two groups of rats, indicating the participation of the oxygen free radical, superoxide, in the maintenance of the systemic BP in a normotensive state. There were no significant differences between the Zn-deficient diet group and the standard diet group in the normotensive state. eNOS expression and Cu/Zn SOD activity in the aorta were also intact in Zn-deficient normotensive rats. These findings suggest that the 4 wk of Zn deficiency was inadequate to alter systemic blood pressure and focal NO signaling in the normotensive state. Long-term Zn deficiency affects the neuronal, immune, and hematopoietic systems, which contribute to systemic and/or local circulation. However, Zn deficiency alone does not cause hypertension and local vascular dysfunction in the normotensive state.
Similar content being viewed by others
References
B. L. Vallee and K. H. Falchuk, The biochemical basis of zinc physiology, Physiol. Rev. 73, 79–118 (1993).
M. Nodera, H. Yanagisawa, K. Moridaira, et al., Reevaluation of zinc deficiency models, Biomed. Res. Trace Elements 9, 19–24 (1998).
H. Yanagisawa, M. Nodera, and O. Wada, Zinc deficiency aggrabates tubulointerstitial nephropathy caused by ureteral obstruction, Biol. Trace Element Res. 65, 1–6 (1998).
H. Yanagisawa, K. Moridaira, and O. Wada, Zinc deficiency further increases the enhanced expression of endothlin-1 in glomeruli of the obstructed kidney, Kidney Int. 58, 575–586 (2000).
N. Inoue, S. Ramasamy, T. Fukai, et al., Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells, Circ. Res. 79, 32–37 (1996).
A. Mügge, J. H. Elwell, T. E. Peterson, et al., Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity, Am. J. Physiol. 260, C219-C225 (1991).
H. A. Omar, P. D. Cherry, M. P. Mortelliti, et al., Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation, Circ. Res. 69, 601–608 (1991).
S. Moncada, The l-arginine: nitric oxide pathway, Acta Physiol. Scand. 145, 201–227 (1992).
L. J. Ignarro, Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling, Pharmacol. Toxicol. 67, 1–7 (1990).
J. Bauersachs, A. Bouloumié, D. Fraccarollo, et al., Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble gyanylate cyclase expression: role of enhanced vascular superoxide production, Circulation 100, 292–298 (1999).
A. Bouloumié, J. Bauersachs, W. Linz, et al., Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production, Hypertension 30, 934–941 (1997).
N. Kurihara, M. E. Alfie, D. H. Sigmin, et al., Role of nNOS in blood pressure regulation in eNOS null mutant mice, Hypertension 32, 856–861 (1998).
C. G. Schnackenberg, W. J. Welch, and C. S. Wilcox, Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide, Hypertension 32, 59–64 (1998).
H. Ynagagisawa, M. Nodera, Y. Umemori, et al., Role of angiotensin II, endothelin-1, and nitric oxide in HgCl2-induced acute renal failure, Toxicol. Appl. Pharmacol. 152, 315–326 (1998).
P. Chomczynski and N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction, Anal. Biochem. 162, 156–159 (1987).
J. Bauersachs, A. Bouloumié, A. Mülsch, et al., Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression and in superoxide anion production, Cardiovasc. Res. 37, 772–779 (1998).
M. Rocco, E. Neilson, J. Hoyer, et al., Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease, Am. J. Physiol. 262, F679-F686 (1992).
Q. Xie, H. Cho, J. Calaycay, et al., Cloning and characterization of inducible nitric oxide synthase from mouse macrophages, Science 256, 225–228 (1992).
H. Yanagisawa, Z. Jin, N. Kurihara, et al., Increases in glomerular eicosanoid production in rats with bilateral ureteral obstruction are mediated by enhanced enzyme activities of both the cyclooxygenase and 5-lipoxygenase pathways, Proc. Soc. Exp. Biol. Med. 203, 291–296 (1993).
V. K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature (Lond). 227, 680–685 (1970).
C. Nebot, M. Moutet, P. Huet, et al., Spectrophotomeric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol, Anal. Biochem. 214, 442–451 (1993).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, et al., Protein measurement with the Folin phenol reagent, J. Biol. Chem. 193, 265–275 (1951).
J. Tamura, H. Yanagisawa, K. Moridaira, et al., Morphological changes of erythrocytes and reticulocytosis in zinc-deficient rats, Biomed. Res. Trace Elements 10, 116–119 (1999).
D. Yoshida, Y. Ikeda, and S. Nakazawa, Suppression of 9L gliosarcoma growth by copper depletion with copper-deficient diet and d-penicillamine, J. Neuro-Oncol. 17, 91–97 (1993).
H. Dahlheim, C. L. White, J. Rothemund, et al., Effect of zinc depletion on angiotensin I-converting enzyme in arterial walls and plasma of the rat, Miner, Electrolyte Metab. 15, 125–129 (1989).
M. Bergomi, S. Rovesti, M. Vinceti, et al., Zinc and copper status and blood pressure, J. Trace Elements Med. Biol. 11, 166–169 (1997).
L. Taittonen, M. Nuutinen, L. Rasanen, et al., Lack of association between copper, zinc, selenium and blood pressure among healthy children, J. Hum. Hypertens. 11, 429–433 (1997).
E. Nava, G. Noll, and T. F. Lüscher, Increased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertension, Circulation 91, 2310–2313 (1995).
N. D. Vaziri, Z. Ni, and F. Oveisi, Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats, Hypertension 31, 1248–1254 (1998).
T. C. Chou, M. H. Yen, C. Y. Li, et al., Alterations of nitric oxide synthase expression with aging and hypertension in rats, Hypertension 31, 643–648 (1998).
H. F. Galley, J. Thornton, P. D. Howdle, et al., Combination oral antioxidant supplementation reduces blood pressure. Clin. Sci. (Lond). 92, 361–365 (1997).
D. W. Laight, A. V. Kaw, M. J. Carrier, et al., Interaction between superoxide anion and nitric oxide in the regulation of vascular endothelial function, Br. J. Pharmacol. 124, 238–244 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sato, M., Kurihara, N., Moridaira, K. et al. Dietary Zn deficiency does not influence systemic blood pressure and vascular nitric oxide signaling in normotensive rats. Biol Trace Elem Res 91, 157–171 (2003). https://doi.org/10.1385/BTER:91:2:157
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:91:2:157