Abstract
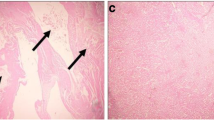
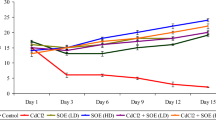
Adhatoda vasica Nees (Acanthaceae) that is used by Ayurvedic physicians possesses some established medicinal properties. Environmental and occupational exposure with cadmium affects the renal system adversely. Cadmium is an established genotoxic agent. In the present study, we evaluated the antioxidant and anticlastogenic efficacy of A. vasica against cadmium chloride (CdCl2)-induced renal oxidative stress and genotoxicity in Swiss albino mice. A single intraperitoneal dose of CdCl2 (5 mg/kg BW) resulted in significant (p<0.001) increase in chromosomal aberration and micronuclei formation. Oral administration of A. vasica at two doses (50 and 100 mg/kg BW) for seven consecutive days showed significant (p<0.001) suppression of mutagenic effects of CdCl2 in plant-pretreated groups. To study the mechanism by which A. vasica exerts its antimutagenic potential, enzymes involved in metabolism and detoxification were also estimated. Cadmium intoxication altered the antioxidant levels and enhanced MDA formation significantly (p<0.001). A. vasica showed significant (p<0.001) recovery in antioxidant status, viz., GSH content, its dependent enzymes, and catalase activity. Prophylactic pretreatment of A. vasica extract in cadmium-intoxicated mice showed marked (p<0.001) inhibition of lipid peroxidation (LPO) and xanthine oxidase (XO) activity. The present findings support that antimutagenic efficacy of A. vasica can be attributed to its restoring effects on antioxidant status and suppression of MDA level formation.
Similar content being viewed by others
References
W. C. Prozialeck and P. C. Lamar, Surface binding and uptake of cadmium (Cd2+) by LLC-PK cells on permeable membrane supports, Arch. Tox. 67, 113–119 (1993).
T. Dalton, K. Fu, B. G. C. Enders, R. D. Palmiter, and G. K. Andrews, Analysis of the effect of over expression of metallothionein-I in transgenic mice on the reproductive toxicity of cadmium, Environ. Health Perspect. 104 (1), 68–71 (1996).
J. M. Peters, D. Thomas, H. Falk, G. Oberdorster, and T. J. Smith, Contribution of metals to respiratory cancer, Environ. Health Perspect. 70, 71–83 (1986).
G. F. Nordberg, and M. Nordberg, in Biological Monitoring of Trace Metals, Vol. 151, T. W. Clarkson, L. Friberg, G. F. Nordberg, and P. R Sager, eds., Plenum Press, New York, 1988.
D. Manca, A. C. Ricard, B. Trottier, and G. Chevalier, Studies on lipidperoxidation in rat tissues following administration of low and moderate doses of cadmium chloride, Toxicology 67, 303–323 (1991).
A. Hartwig Carcinogenicity of metal compounds: possible role of DNA repair inhibition, Toxicol. Lett. 102–103, 235–239 (1998).
G. C. Jagetia and S. K. Adiga, Cadmium chloride induces dose-dependent increases in the frequency of micronuclei in mouse bone marrow, Mut. Res. 306(1), 85–90 (1994).
K. A. Steinmetz and J. D. Potter, Vegetables, fruits and cancer I. epidemiology, Cancer Causes and Control 2, 325–357 (1991).
J. M. Grange and N. J, Snell, Activity of bromohexine and ambroxol, semisynthetic derivatives of vasicine from the Indian shrub Adhatoda vasica, against Mycobacterium tuberculosis in vitro. J. Ethnopharmacol. 5091, 49–53 (1996).
P. K. Patel and H. Venkatakrishna-Bhatt. In vitro study of antimicrobial activity of Adhatoda vasica (leaf extract) on gingival inflammation a preliminary report, Ind. J. Med. Sci. 38(4), 70–72 (1984).
A. Shinawie, Wonder drugs of medicinal plants, Ethnobotany. 29 (2002).
C. K. Atal, Chemistry and Pharmacology of Vasicine: A New Oxytoxic and Abortifacient, RRL, Jammu, India, 1980.
A. Iwashita, K. Hattori, H. Yamamoto, et al. Discovery of quinazolinone and quinoxaline derivatives as potent and selective poly (ADP-ribose) polymerase-1/2 inhibitors, FEBS Lett. 579(6): 1389–1393 (2005).
M. S. M. Rawat, G. Pant, S. Badoni, and Y. S. Negi, Biochemical investigation of some wild fruits of Garhawal Himalayas, Prog. Horticult. 26(1–2), 35–40 (1994).
G. J. Soleas, E. P. Diamandis, and D. M. Goldberg, Wine as a biological fluid: history, production, and role in disease prevention, J. Clin. Lab. Anal. 11, 287–313 (1997).
M. A. Avila, J. A. Velasco, J. Cansado, and V. Notario, Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 54, 2424–2428 (1994).
B. Havsteen, Flavonoids, a class of natural products of high pharmacological potency, Biochem. Pharmacol. 32, 1141–1148 (1983).
W. Schimd, The micronucleus test, Mut. Res. 31, 1–9 (1975).
J. R. Wright, H. D. Colby, P. Miles, and R. Negi, Cytosolic factors which affect microsomal lipid peroxidation in lung and liver, Arch. Biochem. Biophys. 206, 296–304 (1981).
F. Stripe and E. Della Corte, The regulation of rat liver xanthine oxidase, J. Biol. Chem. 244, 3855–3863 (1969).
A. Claiborne, Catalase activity. Methods in Oxygen Radical Research, in R. A. Greenwald, ed. CRC Handbook, CRC Press, Boca Raton, FL, 1985 pp. 283–284.
D. J. Jollow, J. R. Mitchell, N. Zampaglione, and J. R. Gillette, Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic intermediate, Pharmacology 11, 151–169 (1974).
W. H. Habig, M. J. Pubst, and W. B. Jokoby, GST, the first enzymatic step in mercapturic acid formation, J. Biol. Chem. 249, 7130–7139 (1974)
I. Carlberg and B. Mannervik, glutathione reductase levels in rat brain, J. Biol. Chem. 250, 5475–5480 (1975).
J. Mohandas, J.J. Marshall, G. G. Duggin, J. S. Horvath, and D. Tiller, Differential distribution of glutathione and glutathione related enzymes in rabbit kidney, Cancer Res. 44, 5086–5091 (1984).
O. H. Lowry, N. J. Roserough, A. L. Farr, and R. J. Randall, Protein measurement with the phenol reagent, J. Biol. Chem. 193, 2370–2378 (1964).
S. Sultana, S. Ahmed, T. Jahangir, and S. Sharma, Inhibitory effect of celery seeds extract on chemically induced hepatocarcinogenesis: modulation of cell proliferation, metabolism and altered hepatic foci development, Cancer Lett. 221,(1), 11–20 (2005).
R. N. Chopra, Indigenous Drugs of India, Academic Publishers, Calcutta, 1982.
A. H. Amin and D. R. Mehta, Bronchodilator alkaloid from Adhatoda vasica, Nature 184, 1317 (1959).
F. Ali-Osman, Quenching of DNA cross-link precursors of chloroethylnitrosoureas and attenuation of DNA interstrand cross-linking by glutathione, Cancer Res. 49, 5258–5261 (1989).
Paul Talalay and J. W. Fahey. Phytochemicals from cruciferous plants protect against/cancer by modulating carcinogen metabolism, J. Nutr. 131(Suppl), 3027S-3033S (2001).
J. S. Toulitas, L. Neitezel, C. Whitworth, L. P. Rybak, and M. Malafa, Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat, Eur. Arch. Otorhinolaryngol. 257(1), 6–9 (2000).
R. P. Singh, B. Padmavathi, and A. R. Rao, Modulatory influence of Adhatoda vasica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism, antioxidant status, and lipid per oxidation in mice, Mol. Cell Biochem. 213(1–2), 99–109 (2000).
M. Athar and M. Iqbal, Ferric nitrilotriacetate promotes N-diethyl nitrosamine induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis 19, 1133–1139 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahangir, T., Khan, T.H., Prasad, L. et al. Reversal of cadmium chloride-induced oxidative stress and genotoxicity by Adhatoda vasica extract in Swiss albino mice. Biol Trace Elem Res 111, 217–228 (2006). https://doi.org/10.1385/BTER:111:1:217
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:111:1:217