Abstract
The influence of short-term exposure to lead on the energetic status of erythrocytes in rats is reported in this study. The male Wistar rats selected for this study drank water containing 1% lead(II) acetate and/or intraperitoneal injections of 1 or 2 mg/kg body wt every 4 d starting on the eighth of the experiment, over a period of 1 mo.
The whole-blood lead concentration measured after 4 wk was 1.51–35.31 μg/dL. The concentrations of adenosine, adenosine triphosphates, diphosphates, and monophosphates (ATP, ADP, and AMP), guanine triphosphates, diphosphates and monophosphates (GTP, GDP, and GMP), guanosine (Guo), inosine (Ino), inosine monophosphate (IMP), hypoxantine (Hyp), and nicotinamide dinucleotide and its phosphate (NAD+ and NADP+) were determined by high-performance liquid chromatography (HPLC).
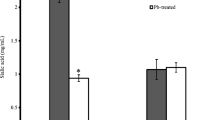
The mean concentrations of ATP, GTP, NAD+, and NADP+ and those of adenylate (AEC) and guanylate (GEC) were significantly reduced in erythrocytes from the animals exposed to lead when compared to untreated controls. These results suggest that a lead ion disrupts the erythrocyte energy pathways. The decreases of NAD+ and ATP could be used as an indicator of the extent of exposure to low levels of lead.
Similar content being viewed by others
References
Institute of Environmental Protection (IEP), Element cycling in the environment, V International Scientific—Technical Conference, Warsaw (2003).
World Health Organization (WHO), Inorganic Lead, Environmental Health Criteria, 165, WHO, Geneva (1995).
T. Dutkiewicz, Report of the Polish Academy of Science, Environmental Toxicology Comittee, Bromat. Chem. Toksykol. 21, 4–20 (1998).
J. Senczuk, Toxicology, 2nd ed., PZWL, Warsaw (2002).
E. Bodak, R. Kolacz, and Z. Dobrzanski, Heavy metals-exposure conditions and defense mechanisms in animals, Med. Weter. 52, 619–626 (1996).
M. L. Caspers, and G. J. Sieger, Inhibition by lead of human erythrocyte (Na+/K+) adenosine triphosphatase associated with binding of 210Pb to membrane fragments, Biochem. Biophys. Acta 600, 27–35 (1980).
Center for Disease Control (CDC), Preventing Lead Poisoning in Young Children, United States Department of Health and Human Services, Department of Health and Human Services, Atlanta, GA (1991).
T. Dutkiewicz, and J. Swiadczak, Lead in environment, Med. Pr. 44, 53–77 (1993).
N. D. Vaziri, C. Y. Lin, F. Farmand, et al., Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension, Kidney Int. 63, 186–194 (2003).
J. Attri, V. Dhawan, S. Mahmood, et al., Effect of vitamin C supplementation on oxidative DNA damage in an experimental model of lead-induced hypertension. Ann. Nutr. Metab. 47, 294–301 (2003).
S. V. Faraone, and T. Wilens, Does stimulant treatment lead to substance use disorders? J. Clin. Psychiatry. 64 (Suppl. 11), 9–13 (2003).
Q. Zhang, G. R. Bratton, R. K. Agarwal, et al., Lead-induced cell signaling cascades in GT1–7 cells, Brain Res. Bull. 61, 207–217 (2003).
C. Li, R. W. Peoples, and F. F. Weight, Mg 2+ inhibition of ATP-activated current in rat nodose ganglion neurons. J. Neurophysiol. 77, 3391–3395 (1997).
Y. W. Lin, S. M. Chuang, and J. L. Yang, Persistent activation of ERK1/2 by lead acetate increases nucleotide excision repair synthesis and confers anti-cytotoxicity and antimutagenicity, Carcinogenesis 24, 53–61 (2003).
A. M. Sharifi, S. Baniasadi, M. Jorjani, et al., Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo, Neurosci. Lett. 329, 45–48 (2002).
L. Struzynska, G. Sulkowski, A. Lenkiewicz, et al., Lead stimulates the glutathione system in selective regions of rat brain, Folia Neuropathol. 40, 203–209 (2003).
C. Taupeau, J. Poupon, D. Treton, et al., Lead reduces messenger RNA and protein levels of cytochrome p450 aromatase and estrogen receptor beta in human ovarian granulosa cells. Biol. Reprod. 68, 1982–1988 (2003).
K. Wozniak, and J. Blasiak, In vitro genotoxicity of lead acetate: induction of single and double DNA strand breaks and DNA-protein cross-links, Mutat. Res. 535, 127–139 (2003).
S. W. Yun, and S. Hoyer, Effects of low-level lead on glycolytic and pyruvate dehydrogenase of rat brain in vitro: relevance to sporadic Alzheimer's disease?, J. Neural Transm. 107, 355–368 (2000).
T. J. B. Simons, Passive transport and binding of lead by human red blood cells, J. Physiol. 378, 267–286 (1986).
T. J. B. Simons, Lead transport and binding by human erythrocytes in vitro, Pflugers Arch. 423, 307–313 (1993).
T. J. B. Simons, Lead-calcium interactions in cellular lead toxicity, Neurotoxicology 14, 97–102 (1993).
I. Baranowska-Bosiacka, A. J. Hlynczak, and B. Machalinski, The impact of lead ions on metabolism of erythrocytes, Med. Pr. 1, 60–65 (2000).
A. J. Simon, and E. S. Hudes, Relationship of ascorbic acid to blood lead levels, JAMA 281, 2289–2293 (1999).
A. Skoczynska, and R. Smolik, The effects of combined exposure to lead and cadmium on serum lipids and lipid peroxides level in rats, Int. J. Occup. Med. Environ. Health. 7, 263–271 (1994).
Z. B. Din, and J. M. Brooks Use of adenylate energy charge as a physiological indicator in toxicity experiments, Bull. Environ. Contamin Toxicol. 36, 1–8 (1986).
M. Grabowska, and M. Guminska, The effect of lead on lactate formation, ATP level and membrane ATPase activities in human erythrocytes in vitro, Int. J. Occup. Med. Environ. Health 9, 265–274 (1996).
M. Hirata, T. Yoshida, K. Miyajima, et al., Correlation between lead in plasma and other indicators of lead exposure among lead-exposed workers, Int. Arch. Occup. Environ. Health 68, 58–63 (1995).
R. Harkness, R. J. Simmonds, and S. B. Coade, Purine transport and metabolism in man: the effect of exercise on concentration of purine bases, nucleosides and nucleotides in plasma, urine, leukocytes and erythrocytes, Clin. Sci. 64, 333–340 (1983).
I. A. Berghdal, A. Schutz, L. Gerhardsson, et al., Lead concentrations in plasma, urine and whole blood, Scand. J. Work Environ. Health. 23, 359–363 (1997).
J. Brandys (ed.), Toxicology, Jagiellonian University Press, Krakow (1999).
K. Kuliczkowski, The effect of lead on red cells, Med. Pr. 31, 143–147 (1980).
J. R. Behari, Determination of lead in blood, Int. J. Environ. Anal. Chem. 10, 149–154 (1981).
R. Smolenski, D. R. Lachno, S. J. M. Ledingham, et al., Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation, J. Chromatogr. 527, 414–420 (1990).
F. I. Ataullakhanov, S. V. Komarova, and V. M. Vitvitsky, A possible role of adenylate metabolism in human erythrocytes: simple matematical model., J. Theor. Biol. 179, 75–86 (1996).
F. I. Ataullakhanov, S. V. Komarova, M. V. Martynov, et al., A possible role of adenylate metabolism in human erythrocytes: simple matematical model. 2. Adenylate metabolism is able to improve the erythrocyte volume stabilisation, J. Theor. Biol. 183, 307–316 (1996).
F. I. Ataullakhanov, V. M. Vitvitsky, S. V. Komarova, et al., Energy-dependent processes and adenylate metabolism in human erythrocytes, Biochemistry (Moscow) 61, 197–203 (1996).
D. E. Paglia, W. N. Valentine, M. Nakatani, et al., Mechanisms of adenosine 5 monophosphate catabolism in human erythrocytes. Blood 67, 988–992 (1986).
M. Erecinska, and D. F. Wilson, Regulation of cellular energy metabolism, J. Membr. Biol. 70, 1–14 (1982).
Z. Jozwiak, Adenine nucleotide in the regulation of erythrocyte structure and metabolism, Post. Hig. Med. Dosw. 39, 1084–1085 (1985).
A. Laurence, J. A. Duley, H. A. Simonds, et al., Characteristic changes in erythrocyte nucleotites in haemolytic anemia with basophilic stippling of differeing aetiology, Cell. Mol. Lett. 4, 406 (1999) (abstract).
K. R. Tanaka, and C. R. Zerez, Red cells enzymopathies of glycolytic pathway, Semin. Hematol. 27, 165–185 (1990).
D. E. Pagliuca, G. L. Mufti, D. Baldwin, et al., Lead poisoning: clinical, biochemical and haematological aspects of recent outbreak, J. Clin. Pathol. 43, 277–281 (1990).
S. Regunathan, and R. Sundaresan, Pyruvate metabolism in the brain of young rats intoxicated with organic and inorganic lead. J. Neurochem. 43, 1346–1351 (1984).
N. E. Lachant, A. Tomoda, and K. R. Tanaka, Inhibition of pentose phosphate shut by lead: potential mechanism for hemolysis in lead poisoning, Blood 63, 518–524 (1984).
D. A. Cory-Slechta, B. Weiss, and C. Cox, Delayed behavioraltoxicity of lead with increasing exposure concentration, Toxicol. Appl. Pharmacol. 71, 342–352 (1983).
I. Baranowska-Bosiacka, and A. J. Hlynczak, The effect of lead ions on the energy metabolism of human erythrocytes in vitro, Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 134, 403–416. (2003).
K. Soltaninejad, A. Kebriaeezadeh, B. Minaiee, et al., Biochemical and ultrastructural evidences for toxicity of lead through free radicals in rat brain, Hum. Exp. Toxicol. 22, 417–423 (2003).
S. Tadashi, A. Takaharu, and U. Koich, Accumulation of erythrocyte nucleotides and their pattern in lead workers, Arch. Environ. Health. 45, 273–277 (1990).
D. E. Paglia, W. N. Valentine, M. Nakatani, et al., Mechanisms of adenosine 5 monophosphate catabolism in human erythrocytes, Blood 67, 988–992 (1986).
H. Holzhutter, G. Jacobasch, and A. Bisdorff, Matemathical modelling of metabolic pathways affected by enzyme deficiency. A mathematical model of glicolysis in normal and pyruvate kinase-deficient red blood cells, Eur. J. Biochem. 149, 101–111 (1985).
C. R. Zerez, and K. R. Tanaka, Impaired nicotinamide adenine dinucleotide synthesis in pyruvate kinase deficient human erythrocytes. A mechanism for decreased total NAD content and possible secondary cause of hemolysis. Blood 69, 999–1005 (1987).
C. R. Zerez, M. D. Wong, and K. R. Tanaka, Partial purification and properties of nicotinamide adenine dinucleotide synthase from human erythrocytes: evidence that enzyme activity is a sensitive indicator of lead exposure, Blood 75, 1576–1582 (1990).
A. Dabrowska, Red cell pyruvate kinase maybe controls of oxygen delivery from erythrocyte, Post. Hig. Med. Dosw. 51, 305–318 (1997).
B. Dobrowska-Bouta, L. Struzynska, and U. Rafalowska, Does lead provoke the peroxidation process in rat brain synaptosomes?, Mol. Chem. Neuropathol. 29, 127–139 (1996).
A. Jedryczko, Involvement of free radicals in lead poisoning, Med. Pr 2, 171–175 (1994).
A. Kraus, H. P. Roth, and M. Kirchgessner, Influence of vitamin C, vitamin E and β-carotene on the osmotic fragility and the primary antioxidant system of erythrocytes in zinc-deficient rats, Arch. Anim. Nutr. 50, 257–269 (1997).
S. B. Maggirwar, D. N. Dhanraj, S. M. Somani, et al., Adenosine acts as endogenous activator of the cellular antioxidant defence system, Biochem. Biophys. Res. Commun. 201, 508–515 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baranowska-Bosiacka, I., Hlynczak, A.J. Effect of lead ions on rat erythrocyte purine content. Biol Trace Elem Res 100, 259–273 (2004). https://doi.org/10.1385/BTER:100:3:259
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:100:3:259