Abstract
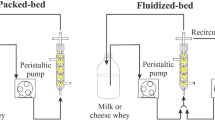
Recombinant β-glycosidase CelB from the hyperthermophilic archaeon Pyrococcus furiosus was produced through expression of the plasmid-encoded gene in Escherichia coli. Bioreactor cultivations of E. coli in the presence of the inductor isopropyl-1-thio-β-d-galactoside (0.1 mM) gave approx 100,000 U of enzyme activity/L of culture medium after 8 h of growth. A technicalgrade enzyme for the hydrolysis of lactose was prepared by precipitating the mesophilic protein at 80°C. A hollow-fiber membrane reactor was developed, and its performance during continuous processing of ultrahigh temperature-treated (UHT) skim milk at 70°C was analyzed regarding long-term stability, productivity, and diffusional limitation thereof. CelB was covalently attached onto Eupergit C in yields of 80%, and a packed-bed immobilized enzyme reactor was used for the continuous hydrolysis of lactose in UHT skim milk at 70°C. The packed-bed reactor was ≈10-fold more stable and gave about the same productivity at 80% substrate conversion as the hollow-fiber reactor at 60% substrate conversion. The marked difference in the stability of free and immobilized CelB seems to reflect mainly binding of the soluble enzyme to the membrane surface of the hollow-fiber module. Under these bound conditions, CelB is essentially inactive. CelB is essentially inactive. Microbial contamination of the reactors did not occur during reaction times of up to 39 d, given that UHT skim milk and not pasteurized skim milk was used as the substrate.
Similar content being viewed by others
References
Cheetham, P. J. S. (1995), in Handbook of Enzyme Biotechnology, 3rd ed., Wiseman, A., ed., Ellis Horwood, London, pp. 419–552.
Pivarnik, L. F., Senecal, A. G. and Rand, A. G. (1995), in Advances in Food and Nutrition Research, vol. 3, Kinsella, J. E. and Taylor, D. L., eds., Academic, NY, pp. 1–101.
Mahoney, R. R. (1985), in Developments in Dairy Chemistry, vol. 3, Fox, P. F., ed., Elsevier Applied Science, Amsterdam, The Netherlands, pp. 69–108.
Gekas, V. and López-Leiva, M. (1985), Process Biochem. 20, 2–12.
Petzelbauer, I., Nidetzky, B., Haltrich, D., and Kulbe, K. D. (1999), Biotechnol. Bioeng. 65, 322–332.
Petzelbauer, I., Zeleny, R., Reiter, A., Kulbe, K.D., and Nidetzky, B. (2000), Biotechnol. Bioeng. 69, 140–149.
Petzelbauer, I., Reiter, A., Splechtna, B., Kosma, P., and Nidetzky, B. (2000), Eur. J. Biochem. 267, 5055–5066.
Lebbink, J. H., Kaper, T., Kengen, S. W., van der Oost, J., and de Vos, W. M. (2001), Methods Enzymol. 330, 364–379.
Fischer, L., Bronmann, R., Kengen, S.W., de Vos, W.M., and Walter, F. (1996), Bio/ Technology 14, 88–91.
Kaper, T., Lebbink, J. H., Pouwels, J., Kopp, J., Schulz, G. E., van der Oost, J., and de Vos, W. M. (2000), Biochemistry 39, 4963–4970.
Petzelbauer, I., Splechtna, B., and Nidetzky, B. (2001), Biotechnol. Bioeng., accepted for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Splechtna, B., Petzelbauer, I., Kuhn, B. et al. Hydrolysis of lactose by β-glycosidase CelB from hyperthermophilic archaeon Pyrococcus furiosus . Appl Biochem Biotechnol 98, 473–488 (2002). https://doi.org/10.1385/ABAB:98-100:1-9:473
Issue Date:
DOI: https://doi.org/10.1385/ABAB:98-100:1-9:473