Abstract
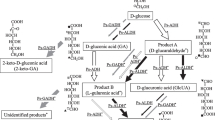
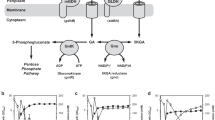
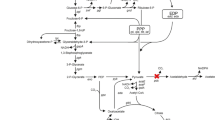
Substrate selectivity of Gluconobacter oxydans (ATCC 9937) for 2,5-diketo-d-gluconic acid (2,5-DKG) production was investigated with glucose, gluconic acid, and gluconolactone in different concentrations using a resting-cell system. The results show that gluconic acid was utilized favorably by G. oxydans as substrate to produce 2,5-DKG. The strain was coupled with glucose dehydrogenase (GDH) and 2,5-DKG reductase for synthesis of 2-keto-l-gulonic acid (2-KLG), a direct precursor of l-ascorbic acid, from glucose. NADP and NADPH were regenerated between GDH and 2,5-DKG reductase. The mole yield of 2-KLG of this multienzyme system was 16.8%. There are three advantages for using the resting cells of G. oxydans to connect GDH with 2,5-DKG reductase for production of 2-KLG: gluconate produced by GDH may immediately be transformed into 2,5-DKG so that a series of problems generally caused by the accumulation of gluconate would be avoided; 2,5-DKG is supplied directly and continuously for 2,5-DKG reductase, so it is unnecessary to take special measures to deal with this unstable substrate as it was in Sonoyama’s tandem fermentation process; and NADP(H) was regenerated within the system without any other components or systems.
Similar content being viewed by others
References
Wakisaka, Y. (1964), Agric. Biol. Chem. 28, 819–827.
Boudrant, J. (1990), Enzyme Microb. Technol. 2, 322–329.
Yin, G. (1986), Chin. J. Biotechnol. 2, 17–21.
Guo, F. and Zheng, X. (1994), Med. Inform. 6, 1–4.
Anderson, S., Berman-Marks, C., Lazarus, R., Miller, J., Stafford, K., Seymour, J., Light, D., Rastetter, W., and Estell, D. (1985), Science 230, 144–149.
Miller, J. V., Estell, D. A., and Lazarus, R. A. (1987), J. Biol. Chem. 262, 9016–9020.
Qazi, G. N., Parshad, R., Verma, V., Chopra, C. L., Buse, R., Trager, M., and Onken, U. (1991), Enzyme Microb. Technol. 13, 504–507.
Sonoyama, T., Tani, H., Matsuda, K., Kageyama, B., Tanimoto, M., Kobayashi, K., Yagi, S., Kyotani, H., and Mitsushima, K. (1982), Appl. Environ. Microbiol. 43, 1064–1069.
Yin, G., Ma, Z., Dong, W., Lin, H., and Ye, Q. (1990), Acta Microbiologica Sinica 31, 198–205.
Cheng, M. and Yan, X. (1981), Pharm. Ind. 12, 15–18.
Fang, X. (1992), in Pharmaceutical Analysis, An, D., Zhang, Z., and Sheng, L., eds., Jinan Publishing House, Jinan, China, pp. 1385, 1386.
Strecker, H. J. (1955), in Methods in Enzymology, vol. 1, Colowich, S. P. and Kaplan, N. O., eds., Academic, London, pp. 335–339.
Li, J., Xiao, N., Yu, R., Yuan, M., Chen, L., Chen, Y., and Chen, L. (1994), in Principle and Methods of Biochemistry Experiments, Beijing University Publishing House, Beijing, pp. 168–170.
Sonoyama, T. and Kobayashi, K. (1987), J. Ferment. Technol. 65, 311–317.
Alcamo, I. E. (1994), in Fundamentals of Microbiology, 4th ed., Benjamin/Cummings, Reading, MA, pp. 123–133.
Dawes, E. A. (1986), in Microbial Energetics, Blackie & Son, Glasgow, UK, pp. 23–39.
Dawes, E. A. (1986), in Microbial Energetics, Blackie & Son, Glasgow, UK, pp. 71–80.
Kragl, U., Kruse, W., Hummel, W., and Wandrey, C. (1996), Biotechnol. Bioeng. 52, 309–319.
Ikemi, M., Koizumi, N., and Ishimatsu, Y. (1990), Biotechnol. Bioeng. 36, 149–165.
Seelbach, K. and Kragl, U. (1997), Enzyme Microb. Technol. 20, 389–392.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, A., Gao, P. Substrate selectivity of Gluconobacter oxydans for production of 2,5-diketo-d-gluconic acid and synthesis of 2-keto-l-gulonic acid in a multienzyme system. Appl Biochem Biotechnol 94, 213–223 (2001). https://doi.org/10.1385/ABAB:94:3:213
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:94:3:213