Abstract
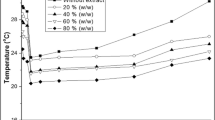
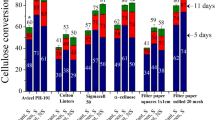
Cellulase could not be selectively collected from fermentation broth by simple foam fractionation, because of the presence of other more surface-active compounds. A new approach of affinity foam fractionation was investigated for improvement. A hardwood hydrolysate (containing cellulose oligomers, substrates to cellulase) and two substrate analogs, i.e., carboxymethyl cellulose (CMC) and xylan hydrolysate, were added before the foaming process. The substrates and substrate analogs were indeed found to bind the cellulase selectively and form more hydrophobic complexes that partition more readily onto bubble surfaces. In this study, the effects of the type and concentration of substrate/analog as well as the presence of cells at different growth stages were examined. The foam fractionation properties evaluated included foaming speed, foam stability, foamate volume, and enrichment of filter paper unit (FPU) and individual cellulase components (i.e., endoglucanases, exoglucanases, and β-glucosidases). Depending on the broth and substrate/analog employed, the foamate FPU could be more than fourfold higher than the starting broth FPU. Addition of substrate/analog also deterred the enrichment of other extracellular proteins, resulting in the desired cellulase purification in the foamate. The value of E/P (enzyme activity-FPU/g/L of proteins) in the foamate reached as high as 18, from a lactose-based fermentation broth with original E/P of 5.6. Among cellulase components, exoglucanases were enriched the most and β-glucosidases the least. The study with CMC of different molecular weights (MW) and degrees of substitution (DS) indicated that the CMC with low DS and high MW performed better in cellulase foam fractionation.
Similar content being viewed by others
References
Montero, G. A., Kirschner, T. F., and Tanner, R. D. (1993), Appl. Biochem. Biotechnol. 39/40, 467–475.
Varley, J. and Ball, S. K. In: Separations for Biotechnology 3, D. L. Pyle, ed., The Royal Society of Chemistry, London, pp. 525–530.
Wenzig, E., Lingg, S., Kerzel, P., Zeh, G., and Mersmann, A. (1993), Chem. Eng. Technol. 16, 405–412.
Batt, C. A. (1991), In: Biomass. In Biotechnology: The Science and the Business, Moses, V., Cape, R. E., eds., Harwood Academic, New York, pp. 521–536.
Bailey, M. J., Askolin, S., Horhammer, N., et al. (2002), Appl. Microbial. Biotechnol. 58(6), 721–727.
Zhang, Q., Lo, C. C., and Ju, L. K. (2005), Bioresour. Technol., submitted.
Dubreil, L., Compoint, J. P., and Marion, D. (1997), J. Agric. Food Chem. 45, 108–118.
Maa, Y. F. and Hsu, C. C. (1997), Biotechnol. Bioeng. 54(6), 503–512.
Bravo Rodríguez, V., Páez Dueñas, P. M., Reyes Requena, A., Aoulad El-Hadj, M., and García López, A. I. (2001), Can. J. Chem. Eng. 79, 289–295.
Sreenath, H. K. (1993), Lebensm-Wisst u-Technol. 26(3), 224–228.
Bravo, V., Páez, M. P., Aould El-Hadj, M., Reyes, A., and García, A. I. (2001), J. Chem. Technol. Biotechnol. 77, 15–20.
Biely, P., Vrsanska, M., and Claeyssens, M. (1991), Eur. J. Biochem. 200, 157–163.
Vladimir, M. C., Olga, V. E., and Anatole, A. K. (1988), Enzyme Microb. Technol. 10, 503–507.
Biely, P. and Markovic, O. (1988), Biotechn. Appl. Biochem. 10(2), 99–106.
Mandels, M., Andreotti, R., and Roche, C. (1976), Biotechnol. Bioeng. Symp. 6, 21–33.
Lee, P. and Moore, M. Abstracts of papers, (2002), 223rd ACS National Meeting, Orlando, FL, United States.
Lo, C.-M., Zhang, Q., and Ju, L. K. (2005), submitted to 27th Symposium on Biotechnology for Fuels and Chemicals.
Miller, W. M., Blanch, H. W., and Wilke, C. R. (1988), Biotechnol. Bioeng. 32, 947–965.
Ohnishi, S. T. and Barr, J. K. (1978), Anal. Biochem. 86, 193–207.
Gunjikar, T. P., Sawant, S. B., and Joshi, J. B. (2001), Biotechnol. Prog. 17, 1166–1168.
Berghem, L. E. R. and Petterson, L. G. (1973), Eur. J. Biochem. 37, 21–30.
Afolabi, O. A. (1997), M.S. Thesis, The University of Akron, Akron, OH.
Klesov, A. A., Rabinoyich, M. L., Sinitsyn, A. P., Churulova, I. V., and Grigorash, S. Y. (1981), Soy. J. Bioorg. Chem. 6, 662–667.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Lo, CM. & Ju, LK. Affinity foam fractionation of Trichoderma cellulase. Appl Biochem Biotechnol 132, 1051–1065 (2006). https://doi.org/10.1385/ABAB:132:1:1051
Issue Date:
DOI: https://doi.org/10.1385/ABAB:132:1:1051