Abstract
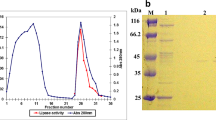
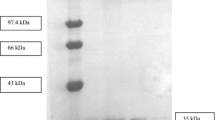
An extracellular lipase was purified from the fermentation broth of Bacillus coagulans ZJU318 by CM-Sepharose chromatography, followed by Sephacryl S-200 chromatography. The lipase was purified 14.7-fold with 18% recovery and a specific activity of 141.1 U/mg. The molecular weight of the homogeneous enzyme was (32 kDa), determined by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. The enzyme activity was maximum at pH 9.0 and was stable over a pH range of 7.0–10.0, and the optimum temperature for the enzyme reaction was 45°C. Little activity loss (6.2%) was observed after 1 h of incubation at 40°C. However, the stability of the lipase decreased sharply at 50 and 60°C. The enzyme activity was strongly inhibited by Ag+ and Cu2+, whereas EDTA caused no inhibition. SDS, Brij 30, and Tween-80 inhibited lipase, whereas Triton X-100 did not significantly inhibit lipase activity.
Similar content being viewed by others
References
Harwood, J. and Macrae, A. R. (1989), Trends Biochem. Sci. 14, 125, 126.
Macrae, A. R. and Hammond, R. C. (1985), Biotechnol. Genet. Eng. Rev. 3, 193–217.
Gupta, R., Gupta, N., and Rathi, P. (2004), Appl. Microbiol. Biotechnol. 64, 763–781.
Kordel, M., Hofmann, B., Schomburg, D., and Schmid, R. D. (1991), J. Bacteriol. 173(15), 4836–4841.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 193, 265–275.
Laemmli, U. K. (1970), Nature, 227, 680–685.
Kambourova, M., Kirilova, N., Mandeva, R., and Derekova, A. (2003), J. Mol. Catal. B Enzym. 22, 307–313.
Kim, H. K., Choi, H. J., Kim, M. H., Sohn, C. B., and Oh, T. K. (2002), Biochim. Biophys. Acta 1583(2), 205–212.
Imamura, S. and Kitaura, S. (2000), J. Biochem. (Tokyo) 127(3), 419–425.
Schmidt-Dannert, C., Sztajer, H., Stocklein, W., Menge, U., and Schmid, R. D. (1994), Biochim. Biophys. Acta 1214(1), 43–53.
Lesuisse, E., Schanck, K., et al. (1993), Eur. J. Biochem. 216(1), 155–160.
Sugihara, A., Tani, T., and Colson, C. (1991), J. Biochem. (Tokyo) 109(2), 211–216
Dosanjh, N. S. and Kaur, J. (2002), Protein Express. Purif. 24(1), 71–75.
Sinchaikul, S., Tyndall, J. D., Fothergill-Gilmore L. A., et al. (2002), Acta Crystallogr. D Biol. Crystallogr. 58, 182–185.
Nawani, N. and Kaur, J. (2000), Mol. Cell. Biochem. 206, 91–96.
Lee, D., Koh, Y., Kim, K., et al. (1999), FEMS Microbiol. Lett. 179(2), 393–400.
Dharmsthiti, S. and Luchai S. (1999), FEMS Microbiol. Lett. 179(2), 241–246.
Kim, H. K., Park, S. Y., Lee, J. K., and Oh, T. K. (1998), Biosci. Biotechnol. Biochem. 62(1), 66–71.
Schmidt-Dannert, C., Rua, M. L., Atomi, H., and Schmid, R. D. (1996), Biochim. Biophys. Acta 1301(1–2), 105–114.
Ghanem, E. H., Al-Sayed, H. A., and Saleh, K. M. (2000), World J. Microbiol. Biotechnol. 16, 459–464.
Stocklein, W., Sztajer, H., Menge, U., and Schmid, R. D. (1993), Biochim. Biophys. Acta 1168, 181–189.
Aisaka, K. and Terada, O. (1981), J. Biochem. 89, 817–822.
Liu, W. H., Beppu, T., and Arima, K. (1973), Agric. Biol. Chem. 37, 2487–2492.
Yadav, R. P., Saxena, R. K., Gupta, R., and Davidson, W. S. (1998), Biotechnol. Appl. Biochem. 28, 243–249.
Litthauer, D., Ginster, A., and van Eeden Skein, E. (2002), Enzyme Microb. Technol. 30, 209–215.
Gilbert, E. J., Cornish, A., and Jones, C. W. (1991), J. Gen. Microbiol. 137, 2223–2229.
Saxena, R. K., Davidson, W. S., Sheoran, A., and Giri, B. (2003), Process Biochem. 39, 239–247.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lianghua, T., Liming, X. Purification and partial characterization of a lipase from Bacillus coagulans ZJU318. Appl Biochem Biotechnol 125, 139–146 (2005). https://doi.org/10.1385/ABAB:125:2:139
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:125:2:139