Abstract
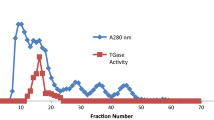
Glutathione reductase was purified from chicken liver and some characteristics of the enzyme were investigated. The purification procedure was composed of four steps: preparation of homogenate, ammonium sulfate precipitation, 2′,5′-ADP Sepharose 4B affinity chromatography, and Sephadex G-200 gel filtration chromatography. Owing to the four consecutive procedures, the enzyme was purified 1714-fold, with a yield of 38%. Specific activity at the final step was 120 enzyme unit (EU)/mg of protein. The purified enzyme showed a single band on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The molecular weight of the enzyme was found to be 100 kDa by Sephadex G-200 gel filtration chromatography, and the subunit molecular weight was found to be 43 kDa by SDS-PAGE. Optimum pH, stable pH, optimum ionic strength, and optimum temperature were 7.0, 7.4, 0.75 M Tris-HCl buffer including 1 mM EDTA, and 50°C, respectively. K M and V max values for NADPH and glutathione disulfide (GSSG) substrates were also determined for the enzyme.
Similar content being viewed by others
References
Gül, M., Kutay, F. Z., Temocin, S., and Hanninen, O. (2000), Indian J. Exp. Biol. 38, 625–634.
Carlberg, I. and Mannervik, B. (1975), J. Biol. Chem. 250, 5475–5480.
Carlberg, I., Altmejd, B., and Mannervik, B. (1981), Biochim. Biophys. Acta 677, 146–152.
Carlberg, I. and Mannervik, B. (1981), Anal. Biochem. 116, 531–536.
Trang, N. L., Bhargava, K. K., and Cerami, A. (1983), Anal. Biochem. 133, 94–99.
Acan, N. L. and Tezcan, E. F. (1989), FEBS Lett. 250, 72–74.
Worthington, D. J. and Rosemeyer, M. A. (1974), Eur. J. Biochem. 48, 167–177.
Krohne-Ehrich, G., Schirmer, R. H., and Untucht-Grau, R. (1977), Eur. J. Biochem. 80, 65–71.
Erat, M., Sakiroĝlu, H., and Çiftçi, M. (2003), Prep. Biochem. Biotechnol. 33, 283–299.
Boggaram, V., Brobjer T., Larson, K., and Mannervik, B. (1979), Anal. Biochem. 98, 335–340.
Mavis, R. D. and Stellwagen, E. (1968), J. Biol. Chem. 243, 809–814.
Rendon, J. L., Calcagno, M., Mendoza-Hernandez, G., and Ondarza, R. N. (1968), Arch. Biochem. Biophys. 248, 215–223.
Lamotte, F., Liaud, N. V., Duviau, M. P., and Kobrehel, K. J. (2000), Agric. Food Chem. 48, 4978–4983.
Halliwell, B. and Foyer, C. H. (1978), Planta 139, 9–17.
Toribio, F., Martinez-Lara, E., Pascual, P., and Lopez-Barea, J. (1996), J. Chromatogr. B, 684, 77–97.
Brodelius, P., Larsson, P. O., and Mosbach, K. (1974), Eur. J. Biochem. 47, 81–89.
Mannervik, B., Jacobsson, K., and Boggaram, V. (1976), FEBS Lett. 66, 221–224.
Andrews, P. (1965), Biochem. J. 96, 595–606.
Carlberg, I. and Mannervik, B. (1985), Methods Enzymol. 113, 484–495.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–251.
Segel, I. H. (1968), Biochemical Calculations, John Wiley and Sons, New York, p. 4003.
Laemmli, U. K. (1970), Nature 227, 680–683.
Lineweaver, H. and Burk, D. (1934), J. Am. Chem. Soc. 57, 685.
Worthington, D. J. and Rosemeyer, M. A. (1975), Eur. J. Biochem. 60, 459–466.
Mize, C. E. and Langdon, R. G. (1962), J. Biol. Chem. 237, 1589–1595.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erat, M., Demir, H. & Šakiroglu, H. Purification of glutathione reductase from chicken liver and investigation of kinetic properties. Appl Biochem Biotechnol 125, 127–138 (2005). https://doi.org/10.1385/ABAB:125:2:127
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:125:2:127