Abstract
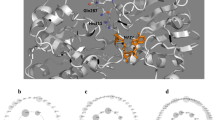
We have previously reported that a chimeric pyrroloquinoline quinone (PQQ) glucose dehydrogenase (GDH), E97A3, which was made up of 97% of Escherichia coli PQQGDH sequence and 3% of Acinetobacter calcoaceticus PQQGDH, showed increased thermal stability compared with both parental enzymes. Site-directed mutagenesis studies were carried out in order to investigate the role of amino-acid substitution at the C-terminal region, Ser 771, of a chimeric PQQGDHs on their thermal stability. A series of Ser 771 substitutions of a chimeric PQQGDH, E99A1, confirmed that hydrophobic interaction governs the thermal stability of the chimeric enzymes. Comparison of the thermal denaturation of E. coli PQQGDH and E97A3 followed by far-ultraviolet (UV) circular dichroism (CD) spectroscopy revealed that E97 A3 acquired stability at the first step of denaturation, which is reversible, and where no significant secondary structure change was observed. These results suggested that the interaction between C-terminal and N-terminal regions may play a crucial role in maintaining the overall structure of β-propeller proteins.
Similar content being viewed by others
References
Matsushita, K., and Adachi, Q. (1993), in Principles and Applications of Quinoproteins, Davidson, V. L. ed., Dekker, New York, pp. 47–63.
Cleton-Jansen, A. M., Goosen, N., Fayet, O., and van de Putte, P. J. (1990), J. Bacteriol. 172, 6308–6315.
Cozier, G. E., and Anthony, C. (1995), Biochem. J. 312, 679–685.
Murzin, A. G. (1992), Proteins 14, 191–201.
Xia, Z., Dai, W., Xiong, J., Hao, Z., Davidson, V. L., White, S., and Mathews, F. S. (1992), J. Biol. Chem. 267, 22,289–22,297.
Baker, S. C., Saunders, N. F. W., Willis, A. C., Ferguson, S. J., Hajdu, J., and Fulop, V. (1997), J. Mol. Biol. 269, 440–455.
Wall, M. A., Coleman, D. E., Lee, E., Iniguez-Lluhi, J. A., Posner, B. A., Gilman, A. G., and Sprang, S. R. (1995), Cell 83, 1047–1058.
Renault, L., Nassar, N., Vetter, I., Becker, J., Klebe, C., Roth, M., and Wittinghofer, A. (1998), Nature 392, 97–101.
Li, J., Brick, P., O'hare, M. C., Skarzynski, T., Lloyd, L. F., Curry, V. A., Clark, I. M., Bigg, H. F., Hazleman, B. L., Cawston, T. E., and Blow, D. M. (1995), Structure, 3, 541–549.
Sode, K. and Sano, H. (1994), Biotechnol. Lett. 16, 455–460.
Sode, K., Yoshida, H., Matsumura, K., Kikuchi, T., Watanabe, M., Yasutake, N., Ito, S., and Sano, H. (1995), Biochem. Biophys. Res. Commun. 211, 268–273.
Sode, K., Shimakita, T., Ohuchi, S., and Yamazaki, T. (1996), Biotechnol. Lett. 18, 997–1002.
Sode, K. and Kojima, K. (1997), Biotechnol. Lett. 19, 1073–1077.
Sode, K., Watanabe, K., Ito, S., Matsumura, K., and Kikuchi, T. (1995), FEBS Lett. 364, 325–327.
Sode, K., Witarto, A. B., Watanabe, K., Noda, K., Ito, S., and Tsugawa, W. (1994), Biotechnol. Lett. 16, 1265–1268.
Hashimoto-Gotoh, T., Mizuno, T., Ogasahara, Y., and Nakagawa, M. (1995), Gene 152, 271–275.
Perczel, A., Hollosi, M., Tusnady, G., and Fasman, G. D. (1991), Protein Eng. 4, 669–679.
Park, K., Perczel, A., and Fasman, G. D. (1992), Protein Sci. 1, 1032–1049.
Lau, F. W. and Bowie, J. U. (1997), Biochemistry 36, 5884–5892.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witarto, A.B., Ohtera, T. & Sode, K. Site-directed mutagenesis study on the thermal stability of a chemiric PQQ glucose dehydrogenase and its structural interpretation. Appl Biochem Biotechnol 77, 159–168 (1999). https://doi.org/10.1385/ABAB:77:1-3:159
Issue Date:
DOI: https://doi.org/10.1385/ABAB:77:1-3:159