Abstract
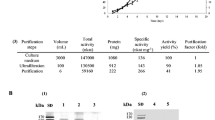
Putrescine oxidase ([PO]; E.C. 1.4.3.4), which catalyzes the oxidative deamination of putrescine into γ-aminobutyraldehyde, has been partially purified from Candida guilliermondii. Among the substrates tested, putrescine has the highest reaction rate, followed by spermidine and cadaverine. The K IN values for putrescine, spermidine, and cadaverine were 20, 200, and 1.1 mM, respectively. The optimum pH and the temperature for PO were 8.0 and 37°C, respectively. Growth of Candida species on putrescine as the solenitrogen source induced the synthesis of PO that converts putrescine into Δ1-pyrroline and γ-aminobutyric acid. These two products were detected and identified from the culture medium. The enzyme was not activated by divalent cations. Among the species of Candida tested, the highest enzyme activity was found in cell-free extracts of C. guilliermondii. The pathway of putrescine degradation was identified by substrate analysis to be along the nonacetylated pathway in C. guilliermondii.
Similar content being viewed by others
References
Tabor, C. W. and Tabor, H. (1976), Annu. Rev. Biochem. 45, 285–306.
Haywood, G. W. and Large, P. J. (1984), J. Gen. Microbiol. 130, 1123–1136.
Haywood, G. W. and Large, P. J. (1985), Eur. J. Biochem. 148, 277–283.
Tabor, C. W. and Tabor, H. (1985), Microbiol. Rev. 49, 81–99.
Large, P. J. (1992), FEMS Microbiol. Rev. 88, 249–262.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Shimizu, E., Tabata, Y., Hayakawa, R., and Yorifuji, T. (1988), Agric. Biol. Chem. 52, 2865–2871.
Yamada, H. (1971), Methods Enzymol. 17B, 726–730.
De Sa, R. J. (1972), J. Biol. Chem. 247, 5527–5534.
Tabor, C. W. and Tabor, H. (1984), Annu. Rev. Biochem. 53, 749–790.
Haywood, G. W. and Large, P. J. (1986), J. Gen. Microbiol. 132, 7–14.
Yamada, H., Isobe, K., and Tani, Y. (1980), Agric. Biol. Chem. 44, 2469–2476.
Ishizuka, H., Horinouchi, S., and Beppu, T. (1993), J. Gen. Microbiol. 139, 425–432.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunasekaran, M., Gunasekaran, U. Partial purification and properties of putrescine oxidase from Candida guilliermondii . Appl Biochem Biotechnol 76, 229–236 (1999). https://doi.org/10.1385/ABAB:76:3:229
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:76:3:229