Abstract
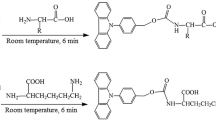
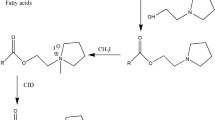
A sensitive method for the determination of free fatty acids using 1,2-benzo-carbazole-9-ethyl-p-toluenesulfonate (BCETS) as tagging reagent with fluorescence detection has been developed. BCETS could easily and quickly label fatty acids in the presence of the K2CO3 catalyst at 80 °C for 30 min in N,N-dimethylformamide solvent. In this study, fatty acids from the extracted Potentilla anserina L. plant sample were sensitively determined. The corresponding derivatives were separated on a reversed-phase Eclipse XDB-C8 column by LC in conjunction with gradient elution. The identification was carried out by post-column APCI-MS in positive-ion detection mode. BCETS-fatty acid derivatives gave an intense molecular ion peak at m/z [M+H]+, the collision-induced dissociation spectra of m/z [M+H]+ produced the specific fragment ions at m/z [M′+CH2CH2]+, m/z 216.6 and m/z [MH−H2O]+ (here, M′: corresponding molecular mass of the fatty acids). The fluorescence excitation and emission wavelengths of the derivatives were at λ ex 279 nm and λ em 380 nm, respectively. Linear correlation coefficients for all fatty acid derivatives are more than 0.9994. Detection limits, at a signal-to-noise ratio of 3:1, are 10.79–34.19 fmol for the labeled fatty acids.




Similar content being viewed by others
References
The Academy of JiangXi New Medicine (1997) Traditional chinese drugs dictionary. Shanghai People Press, Shanghai
Li XY, Yuan HL, Liu YB, Cai GM, Zhang L, Che H (2004) Lishizhen Med Maieria Med Res 15:484–485
Zhao YL, Cai GM, Hong X, Shan LM, Xiao XH (2008) Phytomedicine 15:253–258. doi:10.1016/j.phymed.2008.01.005
Zhang XQ, Zhao YL, Shan LM, Wei ZM, Cai GM (2004) Pharm J Chin People’s Liberation Army 20(4):259–261
Wu ZY (1990) Xinhua herbal scheme. Shanghai Science and Technology Press, Shanghai
Pi L, Hu FZ (2007) Chin Tradit Herbal Drugs 38(11):1625–1627
Wei W, Chen F, Shen AH (2008) Mod Med J Chin 10(1):131–132
Ingalls ST, Minkler PE, Hoppel CL, Nordlander E (1984) J Chromatogr A 299:365–376. doi:10.1016/S0021-9673(01)97852-5
Mukwaya GM, Welch DF (1989) J Clin Microbiol 27:2640–2646
Verken P, van Lint AEM, Bootsma AH, Overmars H, Wanders RJA, van Gennip AH (1998) J Chromatogr B 713:281–287. doi:10.1016/S0378-4347(98)00186-8
Christie WW (1987) HPLC and lipids, 2nd edn. Pergamon Press, Oxford
Voelter W, Huber R, Zech K (1981) J Chromatogr A 217:491–507. doi:10.1016/S0021-9673(00)85107-5
Yoo JS, MacGuffin VL (1992) J Chromatogr A 627:87–96
Takadate A, Masuda T, Tajime C, Murata C, Irikura M, Goya S (1992) Anal Sci 8:663–668
Yamauchi Y, Tomita T, Hirai MS, Terano T, Tamura Y, Yoshida S (1986) J Chromatogr A 357:199–205. doi:10.1016/S0021-9673(01)95821-2
Yoshida T, Uetake A, Yamaguchi H, Nimura N, Kinoshita T (1988) Anal Biochem 173:70–74. doi:10.1016/0003-2697(88)90161-3
Nimura N, Kinoshita T, Yoshida T, Uetake A, Nakai C (1988) Anal Chem 60:2067–2070. doi:10.1021/ac00170a017
Yamaguchi M, Hara S, Matsunaga R, Nakamura M, Ohkura Y (1985) Anal Sci 1:295–296
Iwata T, Inoue K, Nakamura M, Yamaguchi M (1992) Biomed Chromatogr 6:120–123. doi:10.1002/bmc.1130060304
Toyo’oka T, Ishibashi M, Takeda Y, Imai K (1991) Analyst 116:609–613. doi:10.1039/AN9911600609
Toyo’oka T, Ishibashi M, Takeda Y, Nakashima K, Akiyama S, Uzu S, Imai K (1991) J Chromatogr A 588:61–71. doi:10.1016/0021-9673(91)85008-4
Toyo’oka T, Ishibashi M, Terao T, Imai K (1992) Biomed Chromatogr 6:143–148. doi:10.1002/bmc.1130060310
Lu C-Y, Wu H-L, Chen S-H (2000) Chromatographia 51:315–321. doi:10.1007/BF02490609
Yasaka Y, Tanaka M, Shono T, Tetsumi T, Katakawa J (1990) J Chromatogr A 508:133–140. doi:10.1016/S0021-9673(00)91246-9
Akasaka K, Ohrui H, Meguro H (1993) Analyst 118:765. doi:10.1039/AN9931800765
Iwata T, Hirose T, Nakamura M, Yamaguchi M (1994) Analyst 119:1747–1753. doi:10.1039/AN9941901747
Iwata T, Nakamura M, Yamaguchi M (1992) Anal Sci 8:889–892. doi:10.2116/analsci.8.889
Toyo’oka T (2002) Anal Chim Acta 465:111–130. doi:10.1016/S0003-2670(02)00398-7
You J, Shi Y, Ming Y, Yu Z, Yi Y, Liu J (2004) Chromatographia 60:527–535. doi:10.1365/s10337-004-0413-7
Oliveira MM, Salvador MA, Coelho PJ, Carvalho LM (2005) Tetrahedron 61:1681–1691. doi:10.1016/j.tet.2004.12.055
You JM, Ming YF, Shi YW, Zhao XE, Suo YR, Wan HL, Li YL, Sun J (2005) Talanta 68:448–458. doi:10.1016/j.talanta.2005.09.019
Vreken P, van Lint AEM, Bootsma AH, Overmars H, Wanders RJA, van Gennip AH (1998) J Chromatogr B 713:281–287. doi:10.1016/S0378-4347(98)00186-8
Mondello L, Tranchida PQ, Dugo P, Dugo Gi (2006) J Pharm Biomed Anal 41:1566–1570. doi:10.1016/j.jpba.2006.01.027
Slover HT, Lanza E (1979) J Am Oil Chem Soc 56(12):933–943. doi:10.1007/BF02674138
Yang SZ, Wei DZ, Mu BZ (2007) J Biochem Biophys Methods 70:519–523. doi:10.1016/j.jbbm.2007.01.005
Ghasemi E, Yamini Y, Bahramifar N, Sefidkon F (2007) J Food Eng 79:306–311. doi:10.1016/j.jfoodeng.2006.01.059
Yamamoto K, Kinoshita A, Shibahara A (2008) J Chromatogr A 1182:132–135. doi:10.1016/j.chroma.2007.12.071
Acknowledgments
This work was supported by the National Science Foundation of China (No. 20075016) and supported by 100 Talents Programme of The Chinese Academy of Sciences (No. 328).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, L., Song, C., Sun, Z. et al. Determination of Free Fatty Acids in Tibet Folk Medicine Potentilla anserina L. Using a New Labeling Reagent by LC with Fluorescence Detection and Identification with Online Atmospheric Chemical Ionization-MS Identification. Chroma 71, 623–631 (2010). https://doi.org/10.1365/s10337-010-1523-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-010-1523-z