Abstract
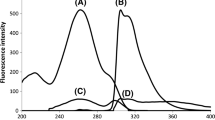
A microchip electrophoresis method with laser induced fluorescence detection was developed for the detection of agmatine (Agm) and octopamine (Oct). The fluorescent derivatization reagent, fluorescein isothiocyanate was used for precolumn derivatization of Agm and Oct. The sodium dodecyl sulfate (SDS) micelles was employed as pseudostationary phase for the separation of Agm and Oct with other endogenous compounds exist in biological samples. Some parameters including buffer concentration, buffer pH, SDS concentration and separation voltage were investigated in detail. Under the optimum conditions, the separation and determination of Agm and Oct was performed within 40 s. The calibration curves were linear for both Agm and Oct over the concentration range of 1.0 × 10−7 to 4.0 × 10−5 M and 1.5 × 10−7 to 4.5 × 10−5 M, respectively. The detection limits of Agm and Oct (S/N = 3) are 5.0 × 10−8 and 8.0 × 10−8 M, respectively. These values make the method very suitable for the determination of Agm and Oct in rat brain tissue and human plasma as demonstrated in this paper.






Similar content being viewed by others
References
Patchett ML, Monk CR, Daniel RM, Morgan HW (1988) J Chromatogr 425:269–276
Kirschbaum J, Rebscher K, Brückner H (2000) J Chromatogr A 881:517–530
Boulton AA, Juorio AV (1982) Brain trace amines. In: Lajtha A (ed) Handbook of neurochemistry, vol 1: chemical and cellular architecture. Plenum Press, New York, p 189
Wang G, Gorbatyuk OS, Dayanithi G, Ouyang W, Wang J, Milner TA, Regunathan S, Reis DJ (2002) Brain Res 932:25–36
Veciana-Nogues TM, Hernandez-Jover T, Marine-Font A, VidalCarou MC (1995) J AOAC Int 78:1045–1050
Reis DJ, Regunathan S (2000) TiPS 21:187–195
Gilad GM, Salame K, Rabey JM, Gilad VH (1996) Life Sci 58:41–46
Gilad GM, Gilad VH (2000) Neurosci Lett 296:97–100
Sombati S, Hoyle G (1984) J Neurobiol 15:481–506
Roeder T (1995) J Pharmacol 114:210–216
Manghani KK, Lunzer M, Billing BH, Sherlock S (1975) Lancet 306:943–946
Capocaccia L, Cangiano C, Attili AF, Angelico M, Cascino A, Rossi Fanelli F (1977) Clin Chim Acta 75:99–105
Boulton AA (1980) Can J Neurol Sci 7:261–263
Kinniburgh DW, Boyd ND (1979) Clin Biochem 12:27–32
D’Andrea G, Terrazzino S, Fortin D, Farruggio A, Rinaldi L, Leon A (2003) Neurosci Lett 346:89–92
Feng Y, Halaris AE, Piletz JE (1997) J Chromatogr B 691:277–286
Zhao S, Feng Y, LeBlanc MH, Piletz JE, Liu YM (2002) Anal Chim Acta 470:155–161
Ibrahim KE, Couch MW, Williams CM (1984) Anal Chem 56:1695–1699
Zhao S, Wang B, Yuan H, Xiao D (2006) J Chromatogr A 1123:138–141
Yu Q, Zhao S, Ye F, Li S (2007) Anal Biochem 369:187–191
Zhao S, Xie C, Lu X, Liu YM (2006) J Chromatogr B 832:52–57
Kvasnicka F, Voldrich M (2006) J Chromatogr A 1103:145–149
Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A (1993) Science 261:895–897
Reyes DR, Iossifidis D, Manz A (2002) Anal Chem 74:2623–2636
Rodríguez I, Lee HK, Li S (1999) Eelectrophoresis 20:118–126
Ro KW, Lim K, Kim H, Hahn JH (2002) Eelectrophoresis 23:1129–1137
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC, Grant No. 20665002), Science Foundation of Ministry of Education, China (No. 206113), and Science Foundation of Guangxi, China (No. 0832004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, M., Huang, Y., Li, X. et al. Detection of Agmatine and Octopamine in Rat Brain and Human Plasma by Microchip Electrophoresis. Chroma 70, 1651–1657 (2009). https://doi.org/10.1365/s10337-009-1387-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1387-2