Abstract
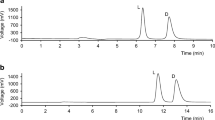
Simple and efficient analytical LC methods using amylose-based stationary phase Chiralpak AS-H were developed for direct enantioseparation of tenatoprazole and several related benzimidazoles. The chromatographic experiments were performed in the normal phase mode with n-hexane–ethanol–triethylamine (TEA) as mobile phase. The effects of the mobile phase additive, concentration of organic modifiers and column temperature were studied for the enantioseparation. The thermodynamic parameters were also calculated from the van’t Hoff plots. It was found that the enantioseparations were all enthalpy driven. The enantiomers of all compounds were resolved (R s > 3.3) within 14 min using n-hexane–ethanol–TEA (20:80:0.1%, v/v/v) as mobile phase with a flow rate of 0.4 mL min−1 at 40 °C. The optimized method was validated for determination of the enantiomers of tenatoprazole in terms of linearity, precision and accuracy according to ICH guidelines and applied to the assay of tenantoprazole bulk drugs. The proposed method was shown to be accurate and suitable for the quantitative determination of tenatoprazole enantiomers.


Similar content being viewed by others
References
Robinson M (2005) Int J Clin Pract 59:709–715
Shin JM, Munson K, Vagin O, Sachs G (2008) Pflugers Arch doi:10.1007/s00424-008-0495-4
Milito DA, Fais S (2005) Future Oncol 1:779–786
Fernandez I, Khiar N (2003) Chem Rev 103:3651–3705
Schwab M, Klotz U, Hofmann U, Schaeffeler E, Leodolter A, Malfertheiner P, Treiber G (2005) Clin Pharmacol Ther 78:627–634
Cotton H, Elebring T, Larsson M, Li L, Sorensen H, Von Unge S (2000) Tetrahedron Asymmetr 11:3819–3825
Hyun MH (2006) J Sep Sci 29:750–761
Ward TJ (2006) Anal Chem 78:3947–3956
Ravikumar M, Narasimhanaidu M, Srinivasulu K, Satyanarayana Raju T, Raja Sekhar Reddy M, Yadagiri Swamy P (2009) Chromatographia 69:163–167
Zhao Y, Pritts WA (2007) J Chromatogr A 1156:228–235
Hiriyanna SG, Basavaiah K, Dhayanithi V, Pati HN (2008) Chromatographia 68:501–505
Miura M, Tada H, Suzuki T (2004) J Chromatogr B 804:389–395
Xie Z, Zhang Y, Xu H, Zhong D (2005) Pharm Res 22:1678–1684
Cass QB, Degani ALG, Cassiano NM, Pedrazolli J Jr (2002) J Chromatogr B 766:153–160
Orlando RM, Bonato PS (2003) J Chromatogr B Analyt Technol Biomed Life Sci 795:227–235
Katsuki H, Yagi H, Arimori K, Nakamura C, Nakano M, Katafuchi S, Fujioka Y, Fujiyama S (1996) Pharm Res 13:611–615
Thunberg L, Hashemi J, Andersson (2008) J Chromatogr B 875:72–80
Belaz KRA, Belaz A, Coimbra M, Barreiro JC, Montanari CA, Cass QB (2008) J Pharm and Biomed Anal 47:81–87
Roberto Cirilli, Ferretti R, Gallinella B, Santis ED, Zanitti L, Torre FL (2008) J Chromatogr A 1177:105–113
Andersson S, Nelander H, Öhlen K (2007) Chirality 19:706–715
Kang JS, Hempel G (2007) Bull Korean Chem Soc 28:1035–1038
Nageswara Rao R, Narasa raju A, Nagaraju D (2006) Talanta 70:805–810
Chankvetadze B, Kartozia I, Yamamoto C, Okamoto Y (2002) J Pharm and Biomed Anal 27:467–478
Cass QB, Lima VV, Oliveira RV, Cassiano NM, Degani ALG, Pedrazzoli J Jr (2003) J Chromatogr B 798:275–281
Blackwell JA, Stringham RW (1997) Anal Chem 69:409–415
O’Brien T, Crocker L, Thompson R, Thompson K, Toma PH, Conlon DA, Feibush B, Moeder C, Bicher G, Grinberg N (1997) Anal Chem 69:1999–2007
Cirill R, Del Giudice MRD, Ferretti R, La Torre F (2001) J Chromatogr A 923:27–36
Acknowledgment
The authors are grateful for financial support from Shenyang Science and Technology Bureau (no. 1063307-1-03) and Liaoning Province Educational Department (2008571), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, J., Li, J., Yan, F. et al. Chiral Separation of Tenatoprazole and Several Related Benzimidazoles by Normal Phase LC Using Amylose-Based Stationary Phase. Chroma 70, 1039–1044 (2009). https://doi.org/10.1365/s10337-009-1296-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1296-4