Abstract
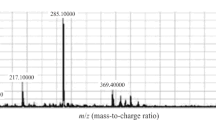
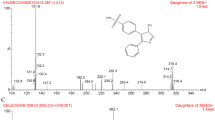
An alternative rapid and sensitive liquid chromatography–tandem mass spectrometry method has been developed and validated for simultaneous analysis of proguanil (PRO) and cycloguanil (CYC) in human plasma. The analytes were extracted from human plasma by solid phase extraction. Riluzole (RIL) was used as an internal standard for proguanil and cycloguanil. A HyPURITY Advance C18 column provided chromatographic separation of analytes followed by detection with mass spectrometry. The method involves simple isocratic chromatography conditions and mass spectrometric detection in the positive ionization mode using an API-4000 system. The proposed method has been validated with linear range of 1.5–150.0 ng mL−1 for PRO and 0.5–50.0 ng mL−1 for CYC. The inter-run and intra-run precision values are within 2.54, 9.19% for PRO and 1.99, 10.69% for CYC at LOQ levels. The overall recoveries for PRO and CYC were 102.52 and 106.72%, respectively. Total elution time was as low as 2.50 min. This validated method was used successfully for analysis of plasma samples from a bioequivalence study.



Similar content being viewed by others
References
GlaxoSmithKline-Malarone (atovaquone and proguanil hydrochloride) tablets prescribing information (2007) 1–4
Wangboonskul J, White NJ, Nosten F, ter Kuile F, Moody RR, Taylor RB (1993) Eur J Clin Pharmacol 44:247–251. doi:10.1007/BF00271366
De Aguiar PF, Heyden YV, Oost YV, Coomber TJ, Massart DL (1997) J Pharm Biomed Anal 15:1781–1787. doi:10.1016/S0731-7085(96)01967-X
Kelly JA, Fletcher KA (1986) J Chromatogr A 381:464–471
Edstein MD (1986) J Chromatogr A 380:184–189
Taylor RB, Moody RR, Oobekpe NA (1987) J Chromatogr A 416:394–399. doi:10.1016/0378-4347(87)80526-1
Bergqvist Y, Funding L, Krysen B, Leek T, Yvell K (1998) Ther Drug Monit 20:325–330. doi:10.1097/00007691-199806000-00014
Taylor RB, Reid RG, Low AS (2001) J Chromatogr A 916:201–206. doi:10.1016/S0021-9673(00)01035-9
Taylor RB, Reid RG (1995) J Pharm Biomed Anal 13:21–26. doi:10.1016/0731-7085(94)00127-N
Bergqvist Y, Funding L, Kaneko A, Krysen B, Leek T (1998) J Chromatogr B Biomed Sci Appl 719:141–149. doi:10.1016/S0378-4347(98)00382-X
Bergqvist Y, Hopstadius C (2000) J Chromatogr B Analyt Technol Biomed Life Sci 741:189–193. doi:10.1016/S0378-4347(00)00082-7
Lejeune D, Souletie I, Houze S, Bricon TL, Bras JL, Gourmel B, Houze P (2007) J Pharm Biomed Anal 43:1106–1115. doi:10.1016/j.jpba.2006.09.036
Ebeshi BU, Obodozie OO, Bolaji OO, Ogunbona FA (2005) Afr J Biotechnol 4:856–861
Hoskins JM, Shenfield GM, Gross AS (1997) J Chromatogr B Biomed Sci Appl 696:81–87. doi:10.1016/S0378-4347(97)00225-9
Taylor RB, Alexander C, Nathwani D, Zimbler N (1996) J Liq Chromatogr Relat Technol 19:1317–1328. doi:10.1080/10826079608006320
Kusaka M, Setiabudy R, Chiba K, Ishizaki T (1996) Am J Trop Med Hyg 54:189–196
Wanwimolruk S, Pratt EL (1995) J Liq Chromatogr 18:4097–4105. doi:10.1080/10826079508013747
Kolawole JA, Taylor RB, Moody RR (1995) J Chromatogr B Biomed Appl 674:149–154. doi:10.1016/0378-4347(95)00293-3
Chaulet FJ, Grelaud G, Patrick MB, Mounier C, Brazier LJ (1994) J Pharm Biomed Anal 12:111–117. doi:10.1016/0731-7085(94)80018-9
Taylor RB, Behrens R, Moody RR (1990) J Chromatogr A 527:490–497
Leveque NL, Charman WN, Chiu FCK (2006) J Chromatogr B Analyt Technol Biomed Life Sci 830:314–321. doi:10.1016/j.jchromb.2005.11.004
Guidance for industry: bioanalytical method validation, U.S. Department of health and human services, food and drug administration centre for drug evaluation and research (CDER), centre for veterinary medicine (CVM) 2001
Acknowledgments
The authors are indebted to Prof. R. T. Sane, Dr. Vikas Vaidya, Mr. Nelson Varghese and Mr. Sudhir Pawar for their continuous support and for providing the laboratory facilities required for this assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pingale, S.G., Nerurkar, K.K., Padgaonkar, A.M. et al. Alternative LC–MS–MS Method for Simultaneous Determination of Proguanil, Its Active Metabolite in Human Plasma and Application to a Bioequivalence Study. Chroma 70, 1095–1102 (2009). https://doi.org/10.1365/s10337-009-1259-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1259-9